 |
PDBsum entry 2c12

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2c12
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Crystal structure of nitroalkane oxidase in complex with spermine, a competitive inhibitor
|
|
Structure:
|
 |
Nitroalkane oxidase. Chain: a, b, c, d, e, f. Engineered: yes
|
|
Source:
|
 |
Fusarium oxysporum. Organism_taxid: 5507. Atcc: 695. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Biol. unit:
|
 |
 Tetramer (from PDB file)
Tetramer (from PDB file)
|
|
Resolution:
|
 |
|
2.07Å
|
R-factor:
|
0.190
|
R-free:
|
0.225
|
|
|
Authors:
|
 |
A.Nagpal,M.P.Valley,P.F.Fitzpatrick,A.M.Orville
|
Key ref:

|
 |
A.Nagpal
et al.
(2006).
Crystal structures of nitroalkane oxidase: insights into the reaction mechanism from a covalent complex of the flavoenzyme trapped during turnover.
Biochemistry,
45,
1138-1150.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
10-Sep-05
|
Release date:
|
01-Feb-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q8X1D8
(NAO_FUSOX) -
Nitroalkane oxidase from Fusarium oxysporum
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
439 a.a.
430 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.7.3.1
- nitroalkane oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
a primary nitroalkane + O2 + H2O = an aldehyde + nitrite + H2O2 + H+
|
|
2.
|
a secondary nitroalkane + O2 + H2O = a ketone + nitrite + H2O2 + H+
|
|
 |
 |
 |
 |
 |
primary nitroalkane
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
 aldehyde
aldehyde
|
+
|
 nitrite
nitrite
|
+
|
 H2O2
H2O2
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
secondary nitroalkane
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
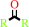 ketone
ketone
|
+
|
 nitrite
nitrite
|
+
|
 H2O2
H2O2
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
45:1138-1150
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of nitroalkane oxidase: insights into the reaction mechanism from a covalent complex of the flavoenzyme trapped during turnover.
|
|
A.Nagpal,
M.P.Valley,
P.F.Fitzpatrick,
A.M.Orville.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Nitroalkane oxidase (NAO) from Fusarium oxysporum catalyzes the oxidation of
neutral nitroalkanes to the corresponding aldehydes or ketones with the
production of H(2)O(2) and nitrite. The flavoenzyme is a new member of the
acyl-CoA dehydrogenase (ACAD) family, but it does not react with acyl-CoA
substrates. We present the 2.2 A resolution crystal structure of NAO trapped
during the turnover of nitroethane as a covalent N5-FAD adduct (ES*). The
homotetrameric structure of ES* was solved by MAD phasing with 52 Se-Met sites
in an orthorhombic space group. The electron density for the
N5-(2-nitrobutyl)-1,5-dihydro-FAD covalent intermediate is clearly resolved. The
structure of ES was used to solve the crystal structure of oxidized NAO at 2.07
A resolution. The c axis for the trigonal space group of oxidized NAO is 485 A,
and there are six subunits (1(1)/(2) holoenzymes) in the asymmetric unit. Four
of the active sites contain spermine (EI), a weak competitive inhibitor, and two
do not contain spermine (E(ox)). The active-site structures of E(ox), EI, and
ES* reveal a hydrophobic channel that extends from the exterior of the protein
and terminates at Asp402 and the N5 position on the re face of the FAD. Thus,
Asp402 is in the correct position to serve as the active-site base, where it is
proposed to abstract the alpha proton from neutral nitroalkane substrates. The
structures for NAO and various members of the ACAD family overlay with
root-mean-square deviations between 1.7 and 3.1 A. The homologous region
typically spans more than 325 residues and includes Glu376, which is the
active-site base in the prototypical member of the ACAD family. However, NAO and
the ACADs exhibit differences in hydrogen-bonding patterns between the
respective active-site base, substrate molecules, and FAD. These likely
differentiate NAO from the homologues and, consequently, are proposed to result
in the unique reaction mechanism of NAO.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
P.F.Fitzpatrick
(2010).
Oxidation of amines by flavoproteins.
|
| |
Arch Biochem Biophys,
493,
13-25.
|
 |
|
|
|
|
 |
A.Héroux,
D.M.Bozinovski,
M.P.Valley,
P.F.Fitzpatrick,
and
A.M.Orville
(2009).
Crystal structures of intermediates in the nitroalkane oxidase reaction.
|
| |
Biochemistry,
48,
3407-3416.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.T.Major,
A.Heroux,
A.M.Orville,
M.P.Valley,
P.F.Fitzpatrick,
and
J.Gao
(2009).
Differential quantum tunneling contributions in nitroalkane oxidase catalyzed and the uncatalyzed proton transfer reaction.
|
| |
Proc Natl Acad Sci U S A,
106,
20734-20739.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Senda,
M.Senda,
S.Kimura,
and
T.Ishida
(2009).
Redox control of protein conformation in flavoproteins.
|
| |
Antioxid Redox Signal,
11,
1741-1766.
|
 |
|
|
|
|
 |
T.Bowles,
A.H.Metz,
J.O'Quin,
Z.Wawrzak,
and
B.F.Eichman
(2008).
Structure and DNA binding of alkylation response protein AidB.
|
| |
Proc Natl Acad Sci U S A,
105,
15299-15304.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Alfieri,
F.Fersini,
N.Ruangchan,
M.Prongjit,
P.Chaiyen,
and
A.Mattevi
(2007).
Structure of the monooxygenase component of a two-component flavoprotein monooxygenase.
|
| |
Proc Natl Acad Sci U S A,
104,
1177-1182.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.F.Fitzpatrick,
D.M.Bozinovski,
A.Héroux,
P.G.Shaw,
M.P.Valley,
and
A.M.Orville
(2007).
Mechanistic and structural analyses of the roles of Arg409 and Asp402 in the reaction of the flavoprotein nitroalkane oxidase.
|
| |
Biochemistry,
46,
13800-13808.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.F.Fitzpatrick
(2007).
Insights into the mechanisms of flavoprotein oxidases from kinetic isotope effects.
|
| |
J Labelled Comp Radiopharm,
50,
1016-1025.
|
 |
|
|
|
|
 |
R.P.Hausinger
(2007).
New insights into acetone metabolism.
|
| |
J Bacteriol,
189,
671-673.
|
 |
|
|
|
|
 |
S.H.Kim,
T.Hisano,
K.Takeda,
W.Iwasaki,
A.Ebihara,
and
K.Miki
(2007).
Crystal structure of the oxygenase component (HpaB) of the 4-hydroxyphenylacetate 3-monooxygenase from Thermus thermophilus HB8.
|
| |
J Biol Chem,
282,
33107-33117.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.Joosten,
and
W.J.van Berkel
(2007).
Flavoenzymes.
|
| |
Curr Opin Chem Biol,
11,
195-202.
|
 |
|
|
|
|
 |
L.De Colibus,
and
A.Mattevi
(2006).
New frontiers in structural flavoenzymology.
|
| |
Curr Opin Struct Biol,
16,
722-728.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

