 |
PDBsum entry 2bk3

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2bk3
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.1.4.3.21
- primary-amine oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a primary methyl amine + O2 + H2O = an aldehyde + H2O2 + NH4+
|
 |
 |
 |
 |
 |
primary methyl amine
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
 aldehyde
aldehyde
|
+
|
 H2O2
H2O2
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.1.4.3.4
- monoamine oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a secondary aliphatic amine + O2 + H2O = a primary amine + an aldehyde + H2O2
|
 |
 |
 |
 |
 |
secondary aliphatic amine
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
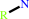 primary amine
primary amine
|
+
|
 aldehyde
aldehyde
|
+
|
 H2O2
H2O2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
280:15761-15766
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Demonstration of isoleucine 199 as a structural determinant for the selective inhibition of human monoamine oxidase B by specific reversible inhibitors.
|
|
F.Hubálek,
C.Binda,
A.Khalil,
M.Li,
A.Mattevi,
N.Castagnoli,
D.E.Edmondson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Several reversible inhibitors selective for human monoamine oxidase B (MAO B)
that do not inhibit MAO A have been described in the literature. The following
compounds: 8-(3-chlorostyryl)caffeine, 1,4-diphenyl-2-butene, and
trans,trans-farnesol are shown to inhibit competitively human, horse, rat, and
mouse MAO B with K(i) values in the low micromolar range but are without effect
on either bovine or sheep MAO B or human MAO A. In contrast, the reversible
competitive inhibitor isatin binds to all known MAO B and MAO A with similar
affinities. Sequence alignments and the crystal structures of human MAO B in
complex with 1,4-diphenyl-2-butene or with trans,trans-farnesol provide
molecular insights into these specificities. These inhibitors span the substrate
and entrance cavities with the side chain of Ile-199 rotated out of its normal
conformation suggesting that Ile-199 is gating the substrate cavity. Ile-199 is
conserved in all known MAO B sequences except bovine MAO B, which has Phe in
this position (the sequence of sheep MAO B is unknown). Phe is conserved in the
analogous position in MAO A sequences. The human MAO B I199F mutant protein of
MAO B binds to isatin (K(i) = 3 microM) but not to the three inhibitors listed
above. The crystal structure of this mutant demonstrates that the side chain of
Phe-199 interferes with the binding of those compounds. This suggests that the
Ile-199 "gate" is a determinant for the specificity of these MAO B
inhibitors and provides a molecular basis for the development of MAO B-specific
reversible inhibitors without interference with MAO A function in
neurotransmitter metabolism.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
FIG. 1. Schematic representation of the MAO B subunit in
complex with trans,trans-farnesol. The FAD-binding domain is in
blue, the substrate-binding domain in red, and the
membrane-binding C-terminal region in green. The FAD cofactor
and trans,trans-farnesol are shown as yellow and black
ball-and-stick representations, respectively. The
inhibitor-binding cavity is outlined by a cyan semitransparent
surface.
|
 |
Figure 2.
FIG. 2. Stereo plots of the complex between
trans,trans-farnesol and wild-type MAO B. A, active site
structure of the bound inhibitor and the conformation of
Ile-199. B, close-up view of the position of bound
trans,trans-farnesol with respect to the flavin ring. The dashed
lines refer to the distances (3.4 Å) between the farnesol
oxygen and the flavin C(4a) position and between the C(1) of
farnesol and the flavin N(5) position.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2005,
280,
15761-15766)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.Aldeco,
B.K.Arslan,
and
D.E.Edmondson
(2011).
Catalytic and inhibitor binding properties of zebrafish monoamine oxidase (zMAO): comparisons with human MAO A and MAO B.
|
| |
Comp Biochem Physiol B Biochem Mol Biol,
159,
78-83.
|
 |
|
|
|
|
 |
J.Wang,
and
D.E.Edmondson
(2010).
High-level expression and purification of rat monoamine oxidase A (MAO A) in Pichia pastoris: comparison with human MAO A.
|
| |
Protein Expr Purif,
70,
211-217.
|
 |
|
|
|
|
 |
A.K.Upadhyay,
and
D.E.Edmondson
(2009).
Development of spin-labeled pargyline analogues as specific inhibitors of human monoamine oxidases A and B.
|
| |
Biochemistry,
48,
3928-3935.
|
 |
|
|
|
|
 |
D.E.Edmondson,
C.Binda,
J.Wang,
A.K.Upadhyay,
and
A.Mattevi
(2009).
Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases.
|
| |
Biochemistry,
48,
4220-4230.
|
 |
|
|
|
|
 |
E.M.Van der Walt,
E.M.Milczek,
S.F.Malan,
D.E.Edmondson,
N.Castagnoli,
J.J.Bergh,
and
J.P.Petzer
(2009).
Inhibition of monoamine oxidase by (E)-styrylisatin analogues.
|
| |
Bioorg Med Chem Lett,
19,
2509-2513.
|
 |
|
|
|
|
 |
J.Wang,
J.Harris,
D.D.Mousseau,
and
D.E.Edmondson
(2009).
Mutagenic probes of the role of Ser209 on the cavity shaping loop of human monoamine oxidase A.
|
| |
FEBS J,
276,
4569-4581.
|
 |
|
|
|
|
 |
M.Naoi,
and
W.Maruyama
(2009).
Functional mechanism of neuroprotection by inhibitors of type B monoamine oxidase in Parkinson's disease.
|
| |
Expert Rev Neurother,
9,
1233-1250.
|
 |
|
|
|
|
 |
A.K.Upadhyay,
and
D.E.Edmondson
(2008).
Characterization of detergent purified recombinant rat liver monoamine oxidase B expressed in Pichia pastoris.
|
| |
Protein Expr Purif,
59,
349-356.
|
 |
|
|
|
|
 |
M.O.Ogunrombi,
S.F.Malan,
G.Terre'blanche,
N.Castagnoli,
J.J.Bergh,
and
J.P.Petzer
(2008).
Structure-activity relationships in the inhibition of monoamine oxidase B by 1-methyl-3-phenylpyrroles.
|
| |
Bioorg Med Chem,
16,
2463-2472.
|
 |
|
|
|
|
 |
D.E.Edmondson,
C.Binda,
and
A.Mattevi
(2007).
Structural insights into the mechanism of amine oxidation by monoamine oxidases A and B.
|
| |
Arch Biochem Biophys,
464,
269-276.
|
 |
|
|
|
|
 |
D.E.Edmondson,
L.DeColibus,
C.Binda,
M.Li,
and
A.Mattevi
(2007).
New insights into the structures and functions of human monoamine oxidases A and B.
|
| |
J Neural Transm,
114,
703-705.
|
 |
|
|
|
|
 |
F.Cruz,
and
D.E.Edmondson
(2007).
Kinetic properties of recombinant MAO-A on incorporation into phospholipid nanodisks.
|
| |
J Neural Transm,
114,
699-702.
|
 |
|
|
|
|
 |
K.Yelekçi,
O.Karahan,
and
M.Toprakçi
(2007).
Docking of novel reversible monoamine oxidase-B inhibitors: efficient prediction of ligand binding sites and estimation of inhibitors thermodynamic properties.
|
| |
J Neural Transm,
114,
725-732.
|
 |
|
|
|
|
 |
Z.B.Ramadan,
M.L.Wrang,
and
K.F.Tipton
(2007).
Species differences in the selective inhibition of monoamine oxidase (1-methyl-2-phenylethyl)hydrazine and its potentiation by cyanide.
|
| |
Neurochem Res,
32,
1783-1790.
|
 |
|
|
|
|
 |
F.Chimenti,
A.Bolasco,
F.Manna,
D.Secci,
P.Chimenti,
A.Granese,
O.Befani,
P.Turini,
S.Alcaro,
and
F.Ortuso
(2006).
Synthesis and molecular modelling of novel substituted-4,5-dihydro-(1H)-pyrazole derivatives as potent and highly selective monoamine oxidase-A inhibitors.
|
| |
Chem Biol Drug Des,
67,
206-214.
|
 |
|
|
|
|
 |
M.B.Youdim,
D.Edmondson,
and
K.F.Tipton
(2006).
The therapeutic potential of monoamine oxidase inhibitors.
|
| |
Nat Rev Neurosci,
7,
295-309.
|
 |
|
|
|
|
 |
M.Kato,
R.M.Wynn,
J.L.Chuang,
C.A.Brautigam,
M.Custorio,
and
D.T.Chuang
(2006).
A synchronized substrate-gating mechanism revealed by cubic-core structure of the bovine branched-chain alpha-ketoacid dehydrogenase complex.
|
| |
EMBO J,
25,
5983-5994.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Vlok,
S.F.Malan,
N.Castagnoli,
J.J.Bergh,
and
J.P.Petzer
(2006).
Inhibition of monoamine oxidase B by analogues of the adenosine A2A receptor antagonist (E)-8-(3-chlorostyryl)caffeine (CSC).
|
| |
Bioorg Med Chem,
14,
3512-3521.
|
 |
|
|
|
|
 |
L.De Colibus,
M.Li,
C.Binda,
A.Lustig,
D.E.Edmondson,
and
A.Mattevi
(2005).
Three-dimensional structure of human monoamine oxidase A (MAO A): relation to the structures of rat MAO A and human MAO B.
|
| |
Proc Natl Acad Sci U S A,
102,
12684-12689.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

