 |
PDBsum entry 2bd1

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
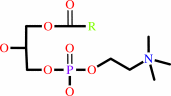 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Acta Crystallogr D Biol Crystallogr
62:717-724
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Suggestive evidence for the involvement of the second calcium and surface loop in interfacial binding: monoclinic and trigonal crystal structures of a quadruple mutant of phospholipase A2.
|
|
K.Sekar,
M.Yogavel,
S.P.Kanaujia,
A.Sharma,
D.Velmurugan,
M.J.Poi,
Z.Dauter,
M.D.Tsai.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structures of the monoclinic and trigonal forms of the quadruple
mutant K53,56,120,121M of recombinant bovine pancreatic phospholipase A2 (PLA2)
have been solved and refined at 1.9 and 1.1 A resolution, respectively.
Interestingly, the monoclinic form reveals the presence of the second calcium
ion. Furthermore, the surface-loop residues are ordered and the conformation of
residues 62-66 is similar to that observed in other structures containing the
second calcium ion. On the other hand, in the trigonal form the surface loop is
disordered and the second calcium is absent. Docking studies suggest that the
second calcium and residues Lys62 and Asp66 from the surface loop could be
involved in the interaction with the polar head group of the membrane
phospholipid. It is hypothesized that the two structures of the quadruple
mutant, monoclinic and trigonal, represent the conformations of PLA2 at the
lipid interface and in solution, respectively. A docked structure with a
phospholipid molecule and with a transition-state analogue bound, one at the
active site coordinating to the catalytic calcium and the other at the second
calcium site, but both at the i-face, is presented.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1 The tertiary structure of the monoclinic form of the
quadruple mutant of recombinant PLA[2]showing the primary (P)
and the secondary (S) calcium ions. The figure was produced
using the program MOLSCRIPT (Kraulis, 1991[Kraulis, P. J.
(1991). J. Appl. Cryst. 24, 946-950.]). For clarity, only
molecule A is shown here.
|
 |
Figure 5.
Figure 5 Ensemble of conformations (99) having minimum docking
energy in the most populated cluster (40%).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the IUCr:
Acta Crystallogr D Biol Crystallogr
(2006,
62,
717-724)
copyright 2006.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

