 |
PDBsum entry 2b03

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
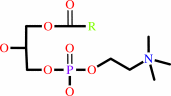 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
369:439-450
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis for bile salt inhibition of pancreatic phospholipase A2.
|
|
Y.H.Pan,
B.J.Bahnson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Bile salt interactions with phospholipid monolayers of fat emulsions are known
to regulate the actions of gastrointestinal lipolytic enzymes in order to
control the uptake of dietary fat. Specifically, on the lipid/aqueous interface
of fat emulsions, the anionic portions of amphipathic bile salts have been
thought to interact with and activate the enzyme group-IB phospholipase A2
(PLA2) derived from the pancreas. To explore this regulatory process, we have
determined the crystal structures of the complexes of pancreatic PLA2 with the
naturally occurring bile salts: cholate, glycocholate, taurocholate,
glycochenodeoxycholate, and taurochenodeoxycholate. The five PLA2-bile salt
complexes each result in a partly occluded active site, and the resulting ligand
binding displays specific hydrogen bonding interactions and extensive
hydrophobic packing. The amphipathic bile salts are bound to PLA2 with their
polar hydroxyl and sulfate/carboxy groups oriented away from the enzyme's
hydrophobic core. The impaired catalytic and interface binding functions implied
by these structures provide a basis for the previous numerous observations of a
biphasic dependence of the rate of PLA2 catalyzed hydrolysis of zwitterionic
glycerophospholipids in the presence of bile salts. The rising or activation
phase is consistent with enhanced binding and activation of the bound PLA2 by
the bile salt induced anionic charge in a zwitterionic interface. The falling or
inhibitory phase can be explained by the formation of a catalytically inert
stoichiometric complex between PLA2 and any bile salts in which it forms a
stable complex. The model provides new insight into the regulatory role that
specific PLA2-bile salt interactions are likely to play in fat metabolism.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. The 2.2 Å crystal structure of pancreatic
group-IB PLA2 complexed with TCDC. The active site residues H48,
D49 and the active site calcium (yellow) are adjacent to the
bile salt bind pocket. The ligand TCDC is displayed in a space
filling view with atoms colored according to the CPK
convention. Apparently, the TCDC bound to the i-face partly
blocks the access route to the active site. The residues K116,
N23, and R6 are shown hydrogen bonded to the taurine-sulfate,
7-hydroxyl, and 3-hydroxyl groups, respectively. Hydrophobic
contacts are shown with F5, I9, F22 and F106. (a) The
PLA2–TCDC complex is displayed with the i-face of the protein
facing the viewer. (b) The i-face of PLA2 is rotated 90°
relative to the view displayed in (a) so that it now faces
down. The space-filling view of TCDC shows the polar hydroxyl
and sulfate groups (red oxygen atoms) facing downward as well.
These views were rendered using the programs MOLSCRIPT,^29
POVSCRIPT^30 and POVRAY [www.povray.org].
|
 |
Figure 7.
Figure 7. Superposition of PLA2s in complex with TCDC with
structures of active site inhibitors MJ33 and MG14. (a) Overlay
of PLA2–TCDC (orange) and PLA2–MJ33^17 (grey; PDB code 1fxf)
structures. (b) Close-up view of (a). (c) Overlay of PLA2–TCDC
(orange) and PLA2–MG14^18 (green; PDB code 1mkv) structures.
(d) Close-up view of (c). These views were rendered using the
programs MOLSCRIPT,^29 POVSCRIPT^30 and POVRAY [www.povray.org].
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2007,
369,
439-450)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.E.Cassidy,
and
W.N.Setzer
(2010).
Cancer-relevant biochemical targets of cytotoxic Lonchocarpus flavonoids: a molecular docking analysis.
|
| |
J Mol Model,
16,
311-326.
|
 |
|
|
|
|
 |
D.De Luca,
A.Minucci,
E.Zecca,
M.Piastra,
D.Pietrini,
V.P.Carnielli,
C.Zuppi,
A.Tridente,
G.Conti,
and
E.D.Capoluongo
(2009).
Bile acids cause secretory phospholipase A2 activity enhancement, revertible by exogenous surfactant administration.
|
| |
Intensive Care Med,
35,
321-326.
|
 |
|
|
|
|
 |
P.Slama,
I.Filippis,
and
M.Lappe
(2008).
Detection of protein catalytic residues at high precision using local network properties.
|
| |
BMC Bioinformatics,
9,
517.
|
 |
|
|
|
|
 |
S.Kojic-Damjanov,
M.Djeric,
M.Mikov,
K.Kuhajda,
and
S.Kevresan
(2008).
Influence of sodium monoketocholate on the hypolipidemic activity of lovastatin in healthy and diabetic rats.
|
| |
Eur J Drug Metab Pharmacokinet,
33,
77-84.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

