 |
PDBsum entry 2art

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.1.20
- lipoate--protein ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-lysyl-[lipoyl-carrier protein] + (R)-lipoate + ATP = N6-[(R)-lipoyl]- L-lysyl-[lipoyl-carrier protein] + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
L-lysyl-[lipoyl-carrier protein]
|
+
|
(R)-lipoate
|
+
|
 ATP
ATP
|
=
|
N(6)-[(R)-lipoyl]- L-lysyl-[lipoyl-carrier protein]
Bound ligand (Het Group name = )
matches with 95.65% similarity
|
+
|
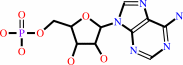 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mg(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
280:38081-38089
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of lipoate-protein ligase A bound with the activated intermediate: insights into interaction with lipoyl domains.
|
|
d.o. .J.Kim,
K.H.Kim,
H.H.Lee,
S.J.Lee,
J.Y.Ha,
H.J.Yoon,
S.W.Suh.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Lipoic acid is the covalently attached cofactor of several multi-component
enzyme complexes that catalyze key metabolic reactions. Attachment of lipoic
acid to the lipoyl-dependent enzymes is catalyzed by lipoate-protein ligases
(LPLs). In Escherichia coli, two distinct enzymes lipoate-protein ligase A
(LplA) and lipB-encoded lipoyltransferase (LipB) catalyze independent pathways
for lipoylation of the target proteins. The reaction catalyzed by LplA occurs in
two steps. First, LplA activates exogenously supplied lipoic acid at the expense
of ATP to lipoyl-AMP. Next, it transfers the enzyme-bound lipoyl-AMP to the
epsilon-amino group of a specific lysine residue of the lipoyl domain to give an
amide linkage. To gain insight into the mechanism of action by LplA, we have
determined the crystal structure of Thermoplasma acidophilum LplA in three
forms: (i) the apo form; (ii) the ATP complex; and (iii) the lipoyl-AMP complex.
The overall fold of LplA bears some resemblance to that of the biotinyl protein
ligase module of the E. coli biotin holoenzyme synthetase/bio repressor (BirA).
Lipoyl-AMP is bound deeply in the bifurcated pocket of LplA and adopts a
U-shaped conformation. Only the phosphate group and part of the ribose sugar of
lipoyl-AMP are accessible from the bulk solvent through a tunnel-like passage,
whereas the rest of the activated intermediate is completely buried inside the
active site pocket. This first view of the activated intermediate bound to LplA
allowed us to propose a model of the complexes between Ta LplA and lipoyl
domains, thus shedding light on the target protein/lysine residue specificity of
LplA.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
FIGURE 1. Electron density of the bound ligands and overall
fold of Ta LplA. A, 2F[o] - F[c] electron density maps of the
bound ligands. Atoms of the ligands are also labeled. B, ribbon
diagram of Ta LplA. Secondary structure elements were assigned
by PROMOTIF (26).  -Helices, -Helices,  -strands,
and loops are colored in red, blue, and yellow, respectively.
Lipoyl-AMP bound near the center of LplA is shown in sticks. All
the figures except Fig. 3 are drawn with PyMOL (DeLano, 2002,
The PyMOL Molecular Graphics System, www.pymol.org). C, topology
diagram of Ta LplA. -strands,
and loops are colored in red, blue, and yellow, respectively.
Lipoyl-AMP bound near the center of LplA is shown in sticks. All
the figures except Fig. 3 are drawn with PyMOL (DeLano, 2002,
The PyMOL Molecular Graphics System, www.pymol.org). C, topology
diagram of Ta LplA.  -Strands are shown as
triangles and -Strands are shown as
triangles and  -helices as circles. D,
stereo C -helices as circles. D,
stereo C  trace of Ta LplA. Every
tenth residue is marked by a black dot, and every twentieth
residue is labeled. Three signature sequence motifs are
highlighted in colored lines: motif I (RRXXGGGXV(F/Y)HD at
positions 71-82) in red, motif II (KhXGXA at positions 145-150)
in green, and motif III (HXX(L/M)LXXX(D/N)LXXLXXhL at positions
161-177) in blue, respectively. trace of Ta LplA. Every
tenth residue is marked by a black dot, and every twentieth
residue is labeled. Three signature sequence motifs are
highlighted in colored lines: motif I (RRXXGGGXV(F/Y)HD at
positions 71-82) in red, motif II (KhXGXA at positions 145-150)
in green, and motif III (HXX(L/M)LXXX(D/N)LXXLXXhL at positions
161-177) in blue, respectively.
|
 |
Figure 2.
FIGURE 2. Lipoyl-AMP binding to the active site. A,
sectional view of the modeled complex showing the target lysine
of the lipoyl domain in the entrance to the lipoyl-AMP binding
pocket of Ta LplA. Note that oxygen atoms of the bound
lipoyl-AMP are surrounded by the positively charged surface
(colored in blue). B, stereo view of the active site around the
bound lipoyl-AMP. Black dotted lines denote hydrogen bonds. Red
balls represent water molecules. C, stereo view of the adenine
ring of the bound lipoyl-AMP and surrounding residues.
Main-chain atoms between Ala^78 and His81 are shown as sticks.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2005,
280,
38081-38089)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Uttamapinant,
K.A.White,
H.Baruah,
S.Thompson,
M.Fernández-Suárez,
S.Puthenveetil,
and
A.Y.Ting
(2010).
A fluorophore ligase for site-specific protein labeling inside living cells.
|
| |
Proc Natl Acad Sci U S A,
107,
10914-10919.
|
 |
|
|
|
|
 |
C.O.Rock
(2009).
Opening a new path to lipoic acid.
|
| |
J Bacteriol,
191,
6782-6784.
|
 |
|
|
|
|
 |
F.A.Hermes,
and
J.E.Cronan
(2009).
Scavenging of cytosolic octanoic acid by mutant LplA lipoate ligases allows growth of Escherichia coli strains lacking the LipB octanoyltransferase of lipoic acid synthesis.
|
| |
J Bacteriol,
191,
6796-6803.
|
 |
|
|
|
|
 |
H.J.Moon,
M.Jeya,
I.S.Yu,
J.H.Ji,
D.K.Oh,
and
J.K.Lee
(2009).
Chaperone-aided expression of LipA and LplA followed by the increase in alpha-lipoic acid production.
|
| |
Appl Microbiol Biotechnol,
83,
329-337.
|
 |
|
|
|
|
 |
M.G.Posner,
A.Upadhyay,
S.Bagby,
D.W.Hough,
and
M.J.Danson
(2009).
A unique lipoylation system in the Archaea. Lipoylation in Thermoplasma acidophilum requires two proteins.
|
| |
FEBS J,
276,
4012-4022.
|
 |
|
|
|
|
 |
M.S.Schonauer,
A.J.Kastaniotis,
V.A.Kursu,
J.K.Hiltunen,
and
C.L.Dieckmann
(2009).
Lipoic acid synthesis and attachment in yeast mitochondria.
|
| |
J Biol Chem,
284,
23234-23242.
|
 |
|
|
|
|
 |
Q.H.Christensen,
and
J.E.Cronan
(2009).
The Thermoplasma acidophilum LplA-LplB complex defines a new class of bipartite lipoate-protein ligases.
|
| |
J Biol Chem,
284,
21317-21326.
|
 |
|
|
|
|
 |
S.Puthenveetil,
D.S.Liu,
K.A.White,
S.Thompson,
and
A.Y.Ting
(2009).
Yeast display evolution of a kinetically efficient 13-amino acid substrate for lipoic acid ligase.
|
| |
J Am Chem Soc,
131,
16430-16438.
|
 |
|
|
|
|
 |
H.Baruah,
S.Puthenveetil,
Y.A.Choi,
S.Shah,
and
A.Y.Ting
(2008).
An engineered aryl azide ligase for site-specific mapping of protein-protein interactions through photo-cross-linking.
|
| |
Angew Chem Int Ed Engl,
47,
7018-7021.
|
 |
|
|
|
|
 |
d.o. .J.Kim,
S.J.Lee,
H.S.Kim,
K.H.Kim,
H.H.Lee,
H.J.Yoon,
and
S.W.Suh
(2008).
Structural basis of octanoic acid recognition by lipoate-protein ligase B.
|
| |
Proteins,
70,
1620-1625.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.M.Keeney,
J.A.Stuckey,
and
M.X.O'Riordan
(2007).
LplA1-dependent utilization of host lipoyl peptides enables Listeria cytosolic growth and virulence.
|
| |
Mol Microbiol,
66,
758-770.
|
 |
|
|
|
|
 |
M.Allary,
J.Z.Lu,
L.Zhu,
and
S.T.Prigge
(2007).
Scavenging of the cofactor lipoate is essential for the survival of the malaria parasite Plasmodium falciparum.
|
| |
Mol Microbiol,
63,
1331-1344.
|
 |
|
|
|
|
 |
S.Günther,
L.Wallace,
E.M.Patzewitz,
P.J.McMillan,
J.Storm,
C.Wrenger,
R.Bissett,
T.K.Smith,
and
S.Müller
(2007).
Apicoplast Lipoic Acid Protein Ligase B Is Not Essential for Plasmodium falciparum.
|
| |
PLoS Pathog,
3,
e189.
|
 |
|
|
|
|
 |
E.Bonilla,
S.Medina-Leendertz,
V.Villalobos,
L.Molero,
and
A.Bohórquez
(2006).
Paraquat-induced oxidative stress in drosophila melanogaster: effects of melatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid.
|
| |
Neurochem Res,
31,
1425-1432.
|
 |
|
|
|
|
 |
Q.Ma,
X.Zhao,
A.Nasser Eddine,
A.Geerlof,
X.Li,
J.E.Cronan,
S.H.Kaufmann,
and
M.Wilmanns
(2006).
The Mycobacterium tuberculosis LipB enzyme functions as a cysteine/lysine dyad acyltransferase.
|
| |
Proc Natl Acad Sci U S A,
103,
8662-8667.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

