 |
PDBsum entry 2aeb

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase/hydrolase inhibitor
|
PDB id
|
|

|

|
2aeb
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.5.3.1
- arginase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Urea Cycle and Arginine Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-arginine + H2O = urea + L-ornithine
|
 |
 |
 |
 |
 |
 L-arginine
L-arginine
|
+
|
H2O
|
=
|
urea
Bound ligand (Het Group name = )
matches with 57.14% similarity
|
+
|
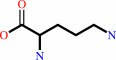 L-ornithine
L-ornithine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
102:13058-13063
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of human arginase I at 1.29-A resolution and exploration of inhibition in the immune response.
|
|
L.Di Costanzo,
G.Sabio,
A.Mora,
P.C.Rodriguez,
A.C.Ochoa,
F.Centeno,
D.W.Christianson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Human arginase I is a potential target for therapeutic intervention in diseases
linked to compromised l-arginine homeostasis. Here, we report high-affinity
binding of the reaction coordinate analogue inhibitors
2(S)-amino-6-boronohexanoic acid (ABH, Kd = 5 nM) and
S-(2-boronoethyl)-l-cysteine (BEC, Kd = 270 nM) to human arginase I, and we
report x-ray crystal structures of the respective enzyme-inhibitor complexes at
1.29- and 1.94-A resolution determined from crystals twinned by hemihedry. The
ultrahigh-resolution structure of the human arginase I-ABH complex yields an
unprecedented view of the binuclear manganese cluster and illuminates the
structural basis for nanomolar affinity: bidentate inner-sphere
boronate-manganese coordination interactions and fully saturated hydrogen bond
networks with inhibitor alpha-amino and alpha-carboxylate groups. These
interactions are therefore implicated in the stabilization of the transition
state for l-arginine hydrolysis. Electron density maps also reveal that
active-site residue H141 is protonated as the imidazolium cation. The location
of H141 is such that it could function as a general acid to protonate the
leaving amino group of l-ornithine during catalysis, and this is a revised
mechanistic proposal for arginase. This work serves as a foundation for studying
the structural and chemical biology of arginase I in the immune response, and we
demonstrate the inhibition of arginase activity by ABH in human and murine
myeloid cells.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Fig. 2. Mechanism of human arginase I. New features
suggested by the 1.29-Å resolution structure of the
complex with ABH are the inner-sphere coordination of  2-NH[2] to 2-NH[2] to
 in
the tetrahedral intermediate, and the protonation of the amino
leaving group of L-ornithine by the conformationally flexible
imidazolium group of general acid H141. D128 may also donate a
proton to L-ornithine before product release. In the final step
of catalysis, the H141 imidazole may serve as a general base to
abstract a proton from the metal-bridging water molecule
(possibly through an intervening solvent molecule). For clarity,
only the side chain guanidinium group of substrate L-arginine
and the side chain amino group of product L-ornithine are
indicated. in
the tetrahedral intermediate, and the protonation of the amino
leaving group of L-ornithine by the conformationally flexible
imidazolium group of general acid H141. D128 may also donate a
proton to L-ornithine before product release. In the final step
of catalysis, the H141 imidazole may serve as a general base to
abstract a proton from the metal-bridging water molecule
(possibly through an intervening solvent molecule). For clarity,
only the side chain guanidinium group of substrate L-arginine
and the side chain amino group of product L-ornithine are
indicated.
|
 |
Figure 4.
Fig. 4. Arginase I-ABH complexes. Summary of average
intermolecular interactions in the human arginase I-ABH complex
(gray numbers) and the rat arginase I-ABH complex (orange
numbers). Note that the higher resolution of the human arginase
I-ABH structure allows for more accurate hydrogen bond distance
measurements. Manganese coordination interactions are indicated
by green dashed lines, and hydrogen bonds are indicated by black
dashed lines. Several slightly shorter and stronger
enzyme-inhibitor hydrogen bond interactions are observed in the
human enzyme, consistent with its enhanced affinity for ABH
(Table 2).
|
 |
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.Riley,
S.C.Roberts,
and
B.Ullman
(2011).
Inhibition profile of Leishmania mexicana arginase reveals differences with human arginase I.
|
| |
Int J Parasitol,
41,
545-552.
|
 |
|
|
|
|
 |
D.Schade,
J.Kotthaus,
and
B.Clement
(2010).
Modulating the NO generating system from a medicinal chemistry perspective: current trends and therapeutic options in cardiovascular disease.
|
| |
Pharmacol Ther,
126,
279-300.
|
 |
|
|
|
|
 |
L.Di Costanzo,
M.Ilies,
K.J.Thorn,
and
D.W.Christianson
(2010).
Inhibition of human arginase I by substrate and product analogues.
|
| |
Arch Biochem Biophys,
496,
101-108.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Y.Shishova,
L.Di Costanzo,
F.A.Emig,
D.E.Ash,
and
D.W.Christianson
(2009).
Probing the specificity determinants of amino acid recognition by arginase.
|
| |
Biochemistry,
48,
121-131.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
G.A.Wells,
I.B.Müller,
C.Wrenger,
and
A.I.Louw
(2009).
The activity of Plasmodium falciparum arginase is mediated by a novel inter-monomer salt-bridge between Glu295-Arg404.
|
| |
FEBS J,
276,
3517-3530.
|
 |
|
|
|
|
 |
J.H.Kim,
L.J.Bugaj,
Y.J.Oh,
T.J.Bivalacqua,
S.Ryoo,
K.G.Soucy,
L.Santhanam,
A.Webb,
A.Camara,
G.Sikka,
D.Nyhan,
A.A.Shoukas,
M.Ilies,
D.W.Christianson,
H.C.Champion,
and
D.E.Berkowitz
(2009).
Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats.
|
| |
J Appl Physiol,
107,
1249-1257.
|
 |
|
|
|
|
 |
J.M.Fitzpatrick,
J.M.Fuentes,
I.W.Chalmers,
T.A.Wynn,
M.Modolell,
K.F.Hoffmann,
and
M.Hesse
(2009).
Schistosoma mansoni arginase shares functional similarities with human orthologs but depends upon disulphide bridges for enzymatic activity.
|
| |
Int J Parasitol,
39,
267-279.
|
 |
|
|
|
|
 |
M.Munder
(2009).
Arginase: an emerging key player in the mammalian immune system.
|
| |
Br J Pharmacol,
158,
638-651.
|
 |
|
|
|
|
 |
V.Sauvé,
P.Roversi,
K.J.Leath,
E.F.Garman,
R.Antrobus,
S.M.Lea,
and
B.C.Berks
(2009).
Mechanism for the hydrolysis of a sulfur-sulfur bond based on the crystal structure of the thiosulfohydrolase SoxB.
|
| |
J Biol Chem,
284,
21707-21718.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
X.Y.Fan,
A.van den Berg,
M.Snoek,
L.G.van der Flier,
B.Smids,
H.M.Jansen,
R.Y.Liu,
and
R.Lutter
(2009).
Arginine deficiency augments inflammatory mediator production by airway epithelial cells in vitro.
|
| |
Respir Res,
10,
62.
|
 |
|
|
|
|
 |
A.Hrabák,
T.Bajor,
and
G.Mészáros
(2008).
The inhibitory effect of various indolyl amino acid derivatives on arginase activity in macrophages.
|
| |
Amino Acids,
34,
293-300.
|
 |
|
|
|
|
 |
D.P.Dowling,
L.Di Costanzo,
H.A.Gennadios,
and
D.W.Christianson
(2008).
Evolution of the arginase fold and functional diversity.
|
| |
Cell Mol Life Sci,
65,
2039-2055.
|
 |
|
|
|
|
 |
K.C.El Kasmi,
J.E.Qualls,
J.T.Pesce,
A.M.Smith,
R.W.Thompson,
M.Henao-Tamayo,
R.J.Basaraba,
T.König,
U.Schleicher,
M.S.Koo,
G.Kaplan,
K.A.Fitzgerald,
E.I.Tuomanen,
I.M.Orme,
T.D.Kanneganti,
C.Bogdan,
T.A.Wynn,
and
P.J.Murray
(2008).
Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens.
|
| |
Nat Immunol,
9,
1399-1406.
|
 |
|
|
|
|
 |
L.Santhanam,
D.W.Christianson,
D.Nyhan,
and
D.E.Berkowitz
(2008).
Arginase and vascular aging.
|
| |
J Appl Physiol,
105,
1632-1642.
|
 |
|
|
|
|
 |
T.Y.Zakharian,
L.Di Costanzo,
and
D.W.Christianson
(2008).
Synthesis of (2S)-2-amino-7,8-epoxyoctanoic acid and structure of its metal-bridging complex with human arginase I.
|
| |
Org Biomol Chem,
6,
3240-3243.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.Di Costanzo,
M.E.Pique,
and
D.W.Christianson
(2007).
Crystal structure of human arginase I complexed with thiosemicarbazide reveals an unusual thiocarbonyl mu-sulfide ligand in the binuclear manganese cluster.
|
| |
J Am Chem Soc,
129,
6388-6389.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.Di Costanzo,
M.Moulin,
M.Haertlein,
F.Meilleur,
and
D.W.Christianson
(2007).
Expression, purification, assay, and crystal structure of perdeuterated human arginase I.
|
| |
Arch Biochem Biophys,
465,
82-89.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.J.Muller,
and
P.A.Scherle
(2006).
Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors.
|
| |
Nat Rev Cancer,
6,
613-625.
|
 |
|
|
|
|
 |
L.Aravind,
L.M.Iyer,
and
E.V.Koonin
(2006).
Comparative genomics and structural biology of the molecular innovations of eukaryotes.
|
| |
Curr Opin Struct Biol,
16,
409-419.
|
 |
|
|
|
|
 |
O.Lespinet,
and
B.Labedan
(2006).
Orphan enzymes could be an unexplored reservoir of new drug targets.
|
| |
Drug Discov Today,
11,
300-305.
|
 |
|
|
|
|
 |
R.Alarcón,
M.S.Orellana,
B.Neira,
E.Uribe,
J.R.García,
and
N.Carvajal
(2006).
Mutational analysis of substrate recognition by human arginase type I--agmatinase activity of the N130D variant.
|
| |
FEBS J,
273,
5625-5631.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

