 |
PDBsum entry 2a7x

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.2.1
- pantoate--beta-alanine ligase (AMP-forming).
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Coenzyme A Biosynthesis (early stages)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(R)-pantoate + beta-alanine + ATP = (R)-pantothenate + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
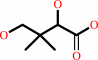 (R)-pantoate
(R)-pantoate
|
+
|
 beta-alanine
beta-alanine
|
+
|
ATP
Bound ligand (Het Group name = )
matches with 50.00% similarity
|
=
|
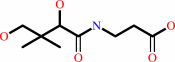 (R)-pantothenate
(R)-pantothenate
|
+
|
AMP
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
45:1554-1561
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of the pantothenate synthetase from Mycobacterium tuberculosis, snapshots of the enzyme in action.
|
|
S.Wang,
D.Eisenberg.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Pantothenate synthetase (PS) from Mycobacterium tuberculosis represents a
potential target for antituberculosis drugs. PS catalyzes the ATP-dependent
condensation of pantoate and beta-alanine to form pantothenate. Previously, we
determined the crystal structure of PS from M. tuberculosis and its complexes
with AMPCPP, pantoate, and pantoyl adenylate. Here, we describe the crystal
structure of this enzyme complexed with AMP and its last substrate,
beta-alanine, and show that the phosphate group of AMP serves as an anchor for
the binding of beta-alanine. This structure confirms that binding of
beta-alanine in the active site cavity can occur only after formation of the
pantoyl adenylate intermediate. A new crystal form was also obtained; it
displays the flexible wall of the active site cavity in a conformation incapable
of binding pantoate. Soaking of this crystal form with ATP and pantoate gives a
fully occupied complex of PS with ATP. Crystal structures of these complexes
with substrates, the reaction intermediate, and the reaction product AMP provide
a step-by-step view of the PS-catalyzed reaction. A detailed reaction mechanism
and its implications for inhibitor design are discussed.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.X.Song,
C.J.Zhou,
X.Guan,
K.H.Sze,
and
H.Y.Hu
(2010).
Solution structure of the N-terminal domain of DC-UbP/UBTD2 and its interaction with ubiquitin.
|
| |
Protein Sci,
19,
1104-1109.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.S.Chakrabarti,
K.G.Thakur,
B.Gopal,
and
S.P.Sarma
(2010).
X-ray crystallographic and NMR studies of pantothenate synthetase provide insights into the mechanism of homotropic inhibition by pantoate.
|
| |
FEBS J,
277,
697-712.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
O.C.Redfern,
B.H.Dessailly,
T.J.Dallman,
I.Sillitoe,
and
C.A.Orengo
(2009).
FLORA: a novel method to predict protein function from structure in diverse superfamilies.
|
| |
PLoS Comput Biol,
5,
e1000485.
|
 |
|
|
|
|
 |
T.R.Ioerger,
and
J.C.Sacchettini
(2009).
Structural genomics approach to drug discovery for Mycobacterium tuberculosis.
|
| |
Curr Opin Microbiol,
12,
318-325.
|
 |
|
|
|
|
 |
A.Ciulli,
D.E.Scott,
M.Ando,
F.Reyes,
S.A.Saldanha,
K.L.Tuck,
D.Y.Chirgadze,
T.L.Blundell,
and
C.Abell
(2008).
Inhibition of Mycobacterium tuberculosis pantothenate synthetase by analogues of the reaction intermediate.
|
| |
Chembiochem,
9,
2606-2611.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Ronconi,
R.Jonczyk,
and
U.Genschel
(2008).
A novel isoform of pantothenate synthetase in the Archaea.
|
| |
FEBS J,
275,
2754-2764.
|
 |
|
|
|
|
 |
J.Seetharamappa,
M.Oke,
H.Liu,
S.A.McMahon,
K.A.Johnson,
L.Carter,
M.Dorward,
M.Zawadzki,
I.M.Overton,
C.A.van Niekirk,
S.Graham,
C.H.Botting,
G.L.Taylor,
M.F.White,
G.J.Barton,
P.J.Coote,
and
J.H.Naismith
(2007).
Purification, crystallization and data collection of methicillin-resistant Staphylococcus aureus Sar2676, a pantothenate synthetase.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
63,
488-491.
|
 |
|
|
|
|
 |
K.L.Tuck,
S.A.Saldanha,
L.M.Birch,
A.G.Smith,
and
C.Abell
(2006).
The design and synthesis of inhibitors of pantothenate synthetase.
|
| |
Org Biomol Chem,
4,
3598-3610.
|
 |
|
|
|
|
 |
R.Jonczyk,
and
U.Genschel
(2006).
Molecular adaptation and allostery in plant pantothenate synthetases.
|
| |
J Biol Chem,
281,
37435-37446.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

