 |
PDBsum entry 2wk5

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Structural features of native human thymidine phosphorylase and in complex with 5-iodouracil
|
|
Structure:
|
 |
Thymidine phosphorylase. Chain: a, b, c, d. Synonym: tdrpase, platelet-derived endothelial cell growth factor, gliostatin, tp, pd-ecgf. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
2.99Å
|
R-factor:
|
0.207
|
R-free:
|
0.284
|
|
|
Authors:
|
 |
E.Mitsiki,A.C.Papageorgiou,S.Iyer,N.Thiyagarajan,S.H.Prior,D.Sleep, C.Finnis,K.R.Acharya
|
|
Key ref:
|
 |
E.Mitsiki
et al.
(2009).
Structures of native human thymidine phosphorylase and in complex with 5-iodouracil.
Biochem Biophys Res Commun,
386,
666-670.
PubMed id:
|
 |
|
Date:
|
 |
|
05-Jun-09
|
Release date:
|
07-Jul-09
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P19971
(TYPH_HUMAN) -
Thymidine phosphorylase from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
482 a.a.
449 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.4.2.4
- thymidine phosphorylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
thymidine + phosphate = 2-deoxy-alpha-D-ribose 1-phosphate + thymine
|
 |
 |
 |
 |
 |
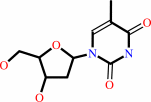 thymidine
thymidine
|
+
|
 phosphate
phosphate
|
=
|
2-deoxy-alpha-D-ribose 1-phosphate
Bound ligand (Het Group name = )
matches with 46.15% similarity
|
+
|
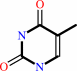 thymine
thymine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochem Biophys Res Commun
386:666-670
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structures of native human thymidine phosphorylase and in complex with 5-iodouracil.
|
|
E.Mitsiki,
A.C.Papageorgiou,
S.Iyer,
N.Thiyagarajan,
S.H.Prior,
D.Sleep,
C.Finnis,
K.R.Acharya.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Thymidine phosphorylase (TP) first identified as platelet derived endothelial
cell growth factor (PD-ECGF) plays a key role in nucleoside metabolism. Human TP
(hTP) is implicated in angiogenesis and is overexpressed in several solid
tumors. Here, we report the crystal structures of recombinant hTP and its
complex with a substrate 5-iodouracil (5IUR) at 3.0 and 2.5A, respectively. In
addition, we provide information on the role of specific residues in the
enzymatic activity of hTP through mutagenesis and kinetic studies.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

