 |
PDBsum entry 2wdv

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2wdv
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|


 588 a.a.
588 a.a.
|
 |

|

|

|

|

|

|

|


 238 a.a.
238 a.a.
|
 |

|

|

|

|

|

|

|


 121 a.a.
121 a.a.
|
 |

|

|

|

|

|

|

|


 105 a.a.
105 a.a.
|
 |

|

|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
E. Coli succinate:quinone oxidoreductase (sqr) with an empty quinone- binding pocket
|
|
Structure:
|
 |
Succinate dehydrogenase flavoprotein subunit. Chain: a, e, i. Engineered: yes. Other_details: fad atom c8m is covalently linked to ne2 of sdha his45. Succinate dehydrogenase iron-sulfur subunit. Chain: b, f, j. Engineered: yes. Succinate dehydrogenase cytochrome b556 subunit.
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Resolution:
|
 |
|
3.20Å
|
R-factor:
|
0.206
|
R-free:
|
0.233
|
|
|
Authors:
|
 |
J.Ruprecht,V.Yankovskaya,E.Maklashina,S.Iwata,G.Cecchini
|
Key ref:

|
 |
J.Ruprecht
et al.
(2009).
Structure of Escherichia coli succinate:quinone oxidoreductase with an occupied and empty quinone-binding site.
J Biol Chem,
284,
29836-29846.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
26-Mar-09
|
Release date:
|
25-Aug-09
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P0AC41
(SDHA_ECOLI) -
Succinate dehydrogenase flavoprotein subunit from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
588 a.a.
588 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P07014
(SDHB_ECOLI) -
Succinate dehydrogenase iron-sulfur subunit from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
238 a.a.
238 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C, D, E, F, G, H, I, J, K, L:
E.C.1.3.5.1
- succinate dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Citric acid cycle
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a quinone + succinate = fumarate + a quinol
|
 |
 |
 |
 |
 |
quinone
Bound ligand (Het Group name = )
matches with 88.89% similarity
|
+
|
 succinate
succinate
|
=
|
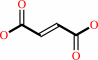 fumarate
fumarate
|
+
|
quinol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD; Iron-sulfur
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
 Iron-sulfur
Iron-sulfur
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
284:29836-29846
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of Escherichia coli succinate:quinone oxidoreductase with an occupied and empty quinone-binding site.
|
|
J.Ruprecht,
V.Yankovskaya,
E.Maklashina,
S.Iwata,
G.Cecchini.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Three new structures of Escherichia coli succinate-quinone oxidoreductase (SQR)
have been solved. One with the specific quinone-binding site (Q-site) inhibitor
carboxin present has been solved at 2.4 A resolution and reveals how carboxin
inhibits the Q-site. The other new structures are with the Q-site inhibitor
pentachlorophenol and with an empty Q-site. These structures reveal important
details unresolved in earlier structures. Comparison of the new SQR structures
shows how subtle rearrangements of the quinone-binding site accommodate the
different inhibitors. The position of conserved water molecules near the quinone
binding pocket leads to a reassessment of possible water-mediated proton uptake
networks that complete reduction of ubiquinone. The dicarboxylate-binding site
in the soluble domain of SQR is highly similar to that seen in high resolution
structures of avian SQR (PDB 2H88) and soluble flavocytochrome c (PDB 1QJD)
showing mechanistically significant structural features conserved across
prokaryotic and eukaryotic SQRs.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Architecture of the dicarboxylate-binding site and ligand in
the carboxin-inhibited structure. The stereo figure shows
residues within 4.0 Å of the ligand TEO, a malate-like
intermediate. Interactions between residues and the ligand are
shown as red dotted lines. The density, shown as a blue mesh, is
a 2mF[o] − DF[c] map, contoured at 1.8 σ. Residues 251–253
and 354 of SdhA have been removed to enable a clearer view of
the binding site.
|
 |
Figure 3.
An unusual cis-peptide bond between Val-A392 and Ser-A393.
The stereo figure shows residues around the cis-peptide (labeled
cP). Polar contacts between residues are shown as red dotted
lines, water molecules as cyan spheres, and a metal ion as a
gray sphere. The density, shown as a blue mesh, is a 2mF[o] −
DF[c] map, contoured at 1.3 σ.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2009,
284,
29836-29846)
copyright 2009.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |

| | |

