 |
PDBsum entry 2v7e

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of coactivator-associated arginine methyltransferase 1 (carm1), unliganded
|
|
Structure:
|
 |
Histone-arginine methyltransferase carm1. Chain: a, b. Fragment: catalytic domain, residues 147-490. Synonym: protein arginine n-methyltransferase 4, coactivator- associated arginine methyltransferase 1. Engineered: yes
|
|
Source:
|
 |
Mus musculus. Mouse. Organism_taxid: 10090. Expressed in: spodoptera frugiperda. Expression_system_taxid: 7108. Expression_system_cell_line: sf9.
|
|
Resolution:
|
 |
|
2.70Å
|
R-factor:
|
0.230
|
R-free:
|
0.276
|
|
|
Authors:
|
 |
W.W.Yue,M.Hassler,S.M.Roe,V.Thompson-Vale,L.H.Pearl
|
Key ref:

|
 |
W.W.Yue
et al.
(2007).
Insights into histone code syntax from structural and biochemical studies of CARM1 methyltransferase.
EMBO J,
26,
4402-4412.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
30-Jul-07
|
Release date:
|
02-Oct-07
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9WVG6
(CARM1_MOUSE) -
Histone-arginine methyltransferase CARM1 from Mus musculus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
608 a.a.
322 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.1.1.319
- type I protein arginine methyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-arginyl-[protein] + 2 S-adenosyl-L-methionine = N(omega),N(omega)- dimethyl-L-arginyl-[protein] + 2 S-adenosyl-L-homocysteine + 2 H+
|
 |
 |
 |
 |
 |
L-arginyl-[protein]
|
+
|
 2
×
S-adenosyl-L-methionine
2
×
S-adenosyl-L-methionine
|
=
|
N(omega),N(omega)- dimethyl-L-arginyl-[protein]
|
+
|
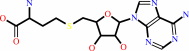 2
×
S-adenosyl-L-homocysteine
2
×
S-adenosyl-L-homocysteine
|
+
|
2
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
EMBO J
26:4402-4412
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Insights into histone code syntax from structural and biochemical studies of CARM1 methyltransferase.
|
|
W.W.Yue,
M.Hassler,
S.M.Roe,
V.Thompson-Vale,
L.H.Pearl.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Coactivator-associated arginine methyltransferase (CARM1) is a transcriptional
coactivator that methylates Arg17 and Arg26 in histone H3. CARM1 contains a
conserved protein arginine methyltransferase (PRMT) catalytic core flanked by
unique pre- and post-core regions. The crystal structures of the CARM1 catalytic
core in the apo and holo states reveal cofactor-dependent formation of a
substrate-binding groove providing a specific access channel for arginine to the
active site. The groove is supported by the first eight residues of the
post-core region (C-extension), not present in other PRMTs. In vitro methylation
assays show that the C-extension is essential for all histone H3 methylation
activity, whereas the pre-core region is required for methylation of Arg26, but
not Arg17. Kinetic analysis shows Arg17 methylation is potentiated by
pre-acetylation of Lys18, and this is reflected in k(cat) rather than K(m).
Together with the absence of specificity subsites in the structure, this
suggests an electrostatic sensing mechanism for communicating the modification
status of vicinal residues as part of the syntax of the 'histone code.'

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2 Crystal structure of CARM1. (A) Left: crystal structure
of CARM1-SAH complex (CARM1[bin]). A CARM1 monomer consists of
the N-domain (blue), C-domain (red), dimerisation arm (yellow)
and C-extension (green). Secondary structure elements are
labelled. Right-top: the crystallised construct (CARM1-  NC)
encompasses aa 147–490. Right-bottom: the two active sites
(arrow) in a CARM1 dimer face towards the central cavity (dotted
circle). (B) Cofactor-binding site of CARM1. The cofactor
product SAH is shown in stick representation, together with the
2F[o]–F[c] electron density map (contoured at 1.5 NC)
encompasses aa 147–490. Right-bottom: the two active sites
(arrow) in a CARM1 dimer face towards the central cavity (dotted
circle). (B) Cofactor-binding site of CARM1. The cofactor
product SAH is shown in stick representation, together with the
2F[o]–F[c] electron density map (contoured at 1.5  )
and residues interacting with SAH. Dotted lines indicate
hydrogen bonds. (C) Conformational changes upon SAH binding.
Superimposition of CARM1[apo] (cyan) and CARM1[bin] (blue)
structures show significant conformational differences in the
Gly-rich loop and helix )
and residues interacting with SAH. Dotted lines indicate
hydrogen bonds. (C) Conformational changes upon SAH binding.
Superimposition of CARM1[apo] (cyan) and CARM1[bin] (blue)
structures show significant conformational differences in the
Gly-rich loop and helix  X.
SAH is shown in stick representation. (D) Surface representation
of the active site in CARM1[apo] (cyan) and CARM1[bin] (blue).
In CARM1[apo], helix X.
SAH is shown in stick representation. (D) Surface representation
of the active site in CARM1[apo] (cyan) and CARM1[bin] (blue).
In CARM1[apo], helix  X
is disordered and the cofactor pocket is accessible. In
CARM1[bin], helix X
is disordered and the cofactor pocket is accessible. In
CARM1[bin], helix  X
(dark blue surface) forms a lid covering the cofactor pocket.
SAH is only accessible via a narrow opening into the putative
arginine pocket. X
(dark blue surface) forms a lid covering the cofactor pocket.
SAH is only accessible via a narrow opening into the putative
arginine pocket.
|
 |
Figure 6.
Figure 6 Surface features of the CARM1 active site. Features of
the active site are coloured: the His415-Asp166 couple (yellow),
C-extension (green) and aromatic residues from Tyr262 in the
double-E hairpin and the PFFRY/THWY motifs (blue). A peptide
sequence of histone H3 (Pro16-Arg17-Lys18) is modelled into the
active site with reference to the structures of PRMT1 and PAD4.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
EMBO J
(2007,
26,
4402-4412)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Y.Yang,
and
M.T.Bedford
(2013).
Protein arginine methyltransferases and cancer.
|
| |
Nat Rev Cancer,
13,
37-50.
|
 |
|
|
|
|
 |
P.Kuhn,
R.Chumanov,
Y.Wang,
Y.Ge,
R.R.Burgess,
and
W.Xu
(2011).
Automethylation of CARM1 allows coupling of transcription and mRNA splicing.
|
| |
Nucleic Acids Res,
39,
2717-2726.
|
 |
|
|
|
|
 |
B.R.Selvi,
K.Batta,
A.H.Kishore,
K.Mantelingu,
R.A.Varier,
K.Balasubramanyam,
S.K.Pradhan,
D.Dasgupta,
S.Sriram,
S.Agrawal,
and
T.K.Kundu
(2010).
Identification of a novel inhibitor of coactivator-associated arginine methyltransferase 1 (CARM1)-mediated methylation of histone H3 Arg-17.
|
| |
J Biol Chem,
285,
7143-7152.
|
 |
|
|
|
|
 |
D.Kim,
J.Lee,
D.Cheng,
J.Li,
C.Carter,
E.Richie,
and
M.T.Bedford
(2010).
Enzymatic activity is required for the in vivo functions of CARM1.
|
| |
J Biol Chem,
285,
1147-1152.
|
 |
|
|
|
|
 |
D.Thomas,
T.M.Lakowski,
M.L.Pak,
J.J.Kim,
and
A.Frankel
(2010).
Förster resonance energy transfer measurements of cofactor-dependent effects on protein arginine N-methyltransferase homodimerization.
|
| |
Protein Sci,
19,
2141-2151.
|
 |
|
|
|
|
 |
S.Carascossa,
P.Dudek,
B.Cenni,
P.A.Briand,
and
D.Picard
(2010).
CARM1 mediates the ligand-independent and tamoxifen-resistant activation of the estrogen receptor alpha by cAMP.
|
| |
Genes Dev,
24,
708-719.
|
 |
|
|
|
|
 |
T.M.Lakowski,
C.Zurita-Lopez,
S.G.Clarke,
and
A.Frankel
(2010).
Approaches to measuring the activities of protein arginine N-methyltransferases.
|
| |
Anal Biochem,
397,
1.
|
 |
|
|
|
|
 |
B.C.Smith,
and
J.M.Denu
(2009).
Chemical mechanisms of histone lysine and arginine modifications.
|
| |
Biochim Biophys Acta,
1789,
45-57.
|
 |
|
|
|
|
 |
K.Kölbel,
C.Ihling,
K.Bellmann-Sickert,
I.Neundorf,
A.G.Beck-Sickinger,
A.Sinz,
U.Kühn,
and
E.Wahle
(2009).
Type I Arginine Methyltransferases PRMT1 and PRMT-3 Act Distributively.
|
| |
J Biol Chem,
284,
8274-8282.
|
 |
|
|
|
|
 |
M.T.Bedford,
and
S.G.Clarke
(2009).
Protein arginine methylation in mammals: who, what, and why.
|
| |
Mol Cell,
33,
1.
|
 |
|
|
|
|
 |
P.Kuhn,
Q.Xu,
E.Cline,
D.Zhang,
Y.Ge,
and
W.Xu
(2009).
Delineating Anopheles gambiae coactivator associated arginine methyltransferase 1 automethylation using top-down high resolution tandem mass spectrometry.
|
| |
Protein Sci,
18,
1272-1280.
|
 |
|
|
|
|
 |
Q.Feng,
B.He,
S.Y.Jung,
Y.Song,
J.Qin,
S.Y.Tsai,
M.J.Tsai,
and
B.W.O'Malley
(2009).
Biochemical control of CARM1 enzymatic activity by phosphorylation.
|
| |
J Biol Chem,
284,
36167-36174.
|
 |
|
|
|
|
 |
T.M.Lakowski,
and
A.Frankel
(2009).
Kinetic analysis of human protein arginine N-methyltransferase 2: formation of monomethyl- and asymmetric dimethyl-arginine residues on histone H4.
|
| |
Biochem J,
421,
253-261.
|
 |
|
|
|
|
 |
K.Fronz,
S.Otto,
K.Kölbel,
U.Kühn,
H.Friedrich,
A.Schierhorn,
A.G.Beck-Sickinger,
A.Ostareck-Lederer,
and
E.Wahle
(2008).
Promiscuous modification of the nuclear poly(A)-binding protein by multiple protein-arginine methyltransferases does not affect the aggregation behavior.
|
| |
J Biol Chem,
283,
20408-20420.
|
 |
|
|
|
|
 |
O.Obianyo,
T.C.Osborne,
and
P.R.Thompson
(2008).
Kinetic mechanism of protein arginine methyltransferase 1.
|
| |
Biochemistry,
47,
10420-10427.
|
 |
|
|
|
|
 |
T.M.Lakowski,
and
A.Frankel
(2008).
A kinetic study of human protein arginine N-methyltransferase 6 reveals a distributive mechanism.
|
| |
J Biol Chem,
283,
10015-10025.
|
 |
|
|
|
|
 |
M.De la Peña,
O.J.Kyrieleis,
and
S.Cusack
(2007).
Structural insights into the mechanism and evolution of the vaccinia virus mRNA cap N7 methyl-transferase.
|
| |
EMBO J,
26,
4913-4925.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Lall
(2007).
Primers on chromatin.
|
| |
Nat Struct Mol Biol,
14,
1110-1115.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

