 |
PDBsum entry 2qqe

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.1.21
- thymidine kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
thymidine + ATP = dTMP + ADP + H+
|
 |
 |
 |
 |
 |
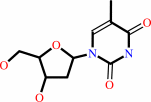 thymidine
thymidine
|
+
|
ATP
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
 dTMP
dTMP
|
+
|
 ADP
ADP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure
15:1555-1566
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Quaternary structure change as a mechanism for the regulation of thymidine kinase 1-like enzymes.
|
|
D.Segura-Peña,
J.Lichter,
M.Trani,
M.Konrad,
A.Lavie,
S.Lutz.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The human cytosolic thymidine kinase (TK) and structurally related TKs in
prokaryotes play a crucial role in the synthesis and regulation of the cellular
thymidine triphosphate pool. We report the crystal structures of the TK
homotetramer from Thermotoga maritima in four different states: its apo-form, a
binary complex with thymidine, as well as the ternary structures with the two
substrates (thymidine/AppNHp) and the reaction products (TMP/ADP). In
combination with fluorescence spectroscopy and mutagenesis experiments, our
results demonstrate that ATP binding is linked to a substantial reorganization
of the enzyme quaternary structure, leading to a transition from a closed,
inactive conformation to an open, catalytic state. We hypothesize that these
structural changes are relevant to enzyme function in situ as part of the
catalytic cycle and serve an important role in regulating enzyme activity by
amplifying the effects of feedback inhibitor binding.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. The TmTK Three-Dimensional Structure Remains
Largely Unchanged upon Substrate Binding
(A) Stereo view of
an overlay of three TmTK backbones in different complexation
states. Apo-form in cyan, in complex with thymidine in yellow
and the ternary complex in magenta. Purple spheres represent the
Mg ion in the active site.
(B) Ribbon representation of the
three individual TmTK structures, using the same color code as
in (A). Yellow spheres mark the position of the structural zinc
atom. The two regions of the protein that undergo a
conformational change upon substrate binding are the lasso loop
(red lines) and the β-hairpin loop (blue lines). The dashed
lines represent parts of the flexible loops that could not be
modeled due to lack of electron density. Most of the lasso loop,
which is involved in thymidine binding, was disorganized in the
apo-form, yet became almost fully defined in the complex with
thymidine, and could be completely modeled in the ground-state
complex (thymidine + AppNHp). The β-hairpin loop composed of
βc1/β3 is sensitive to the presence of phosphoryl donor. Only
in the presence of AppNHp was the electron density for βc1
present.
|
 |
Figure 3.
Figure 3. ATP Induces a Quaternary Structural Rearrangement
in TmTK
(A) Sphere representation of TmTK homotetramer in
the three substrate-binding states. Individual monomers are
shown in different colors. Note that, upon ATP binding, the
tetramer expands due to an increase in the separation between
the monomers that make the interface to which the adenosine
moiety of ATP is bound (weak dimer interface, horizontal
brackets). In contrast the second type of monomer-monomer
interface remains unchanged (vertical brackets). ATP and
thymidine are shown in yellow and green, respectively.
(B)
Stereo view overlay of the TmTK apo-tetramer (cyan) with the
tetramer in complex with thymidine (yellow). The overlay was
done on the monomer A (Ma). There is an excellent superposition
of the two tetrameric structures, indicating the same subunit
organization for the two tetrameric structures.
(C)
Analogous stereoview overlay between the TmTK binary complex
(with thymidine) and the ternary complex (magenta color).
Subunits across the strong dimer interface show an excellent
overlay (Ma and Mb). In constrast, the relative orientation of
the remaining two monomers is changed. Note the change of
orientation between monomers across the weak interface (Md with
respect to Ma and Mc with respect to Mb). Black lines mark the
positions of helix α1 in each tetramer. In the closed state of
the tetramer (binary complex in yellow), helix α1 would clash
with the adenosine moiety of ATP.
|
 |
|
|

|
| |
The above figures are
reprinted
from an Open Access publication published by Cell Press:
Structure
(2007,
15,
1555-1566)
copyright 2007.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.Zander,
M.Hartenfeller,
V.Hähnke,
E.Proschak,
S.Besier,
T.A.Wichelhaus,
and
G.Schneider
(2010).
Multistep virtual screening for rapid and efficient identification of non-nucleoside bacterial thymidine kinase inhibitors.
|
| |
Chemistry,
16,
9630-9637.
|
 |
|
|
|
|
 |
B.Munch-Petersen
(2009).
Reversible tetramerization of human TK1 to the high catalytic efficient form is induced by pyrophosphate, in addition to tripolyphosphates, or high enzyme concentration.
|
| |
FEBS J,
276,
571-580.
|
 |
|
|
|
|
 |
S.Lutz,
L.Liu,
and
Y.Liu
(2009).
Engineering Kinases to Phosphorylate Nucleoside Analogs for Antiviral and Cancer Therapy.
|
| |
Chimia (Aarau),
63,
737-744.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

