 |
PDBsum entry 2jzr

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Electron transport
|
PDB id
|
|

|

|
2jzr
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Electron transport
|
 |
|
Title:
|
 |
Solution structure of the oxidized form (cys67-cys70) of the n- terminal domain of pilb from n. Meningitidis.
|
|
Structure:
|
 |
Peptide methionine sulfoxide reductase msra/msrb. Chain: a. Fragment: thioredoxin domain. Synonym: thioredoxin. Engineered: yes
|
|
Source:
|
 |
Neisseria meningitidis serogroup a. Gene: msrab, pilb. Expressed in: escherichia coli.
|
|
NMR struc:
|
 |
20 models
|
 |
|
Authors:
|
 |
M.Quinternet,P.Tsan,F.Neiers,C.Beaufils,S.Boschi-Muller,M.Averlant- Petit,G.Branlant,M.Cung
|
|
Key ref:
|
 |
M.Quinternet
et al.
(2008).
Solution structure and dynamics of the reduced and oxidized forms of the N-terminal domain of PilB from Neisseria meningitidis.
Biochemistry,
47,
8577-8589.
PubMed id:
|
 |
|
Date:
|
 |
|
15-Jan-08
|
Release date:
|
29-Jul-08
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9JWM8
(MSRAB_NEIMA) -
Peptide methionine sulfoxide reductase MsrA/MsrB from Neisseria meningitidis serogroup A / serotype 4A (strain DSM 15465 / Z2491)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
522 a.a.
144 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.1.8.4.11
- peptide-methionine (S)-S-oxide reductase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
L-methionyl-[protein] + [thioredoxin]-disulfide + H2O = L-methionyl- (S)-S-oxide-[protein] + [thioredoxin]-dithiol
|
|
2.
|
[thioredoxin]-disulfide + L-methionine + H2O = L-methionine (S)-S- oxide + [thioredoxin]-dithiol
|
|
 |
 |
 |
 |
 |
L-methionyl-[protein]
|
+
|
[thioredoxin]-disulfide
|
+
|
H2O
|
=
|
L-methionyl- (S)-S-oxide-[protein]
|
+
|
[thioredoxin]-dithiol
|
|
 |
 |
 |
 |
 |
[thioredoxin]-disulfide
|
+
|
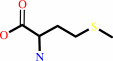 L-methionine
L-methionine
|
+
|
H2O
|
=
|
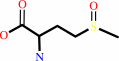 L-methionine (S)-S- oxide
L-methionine (S)-S- oxide
|
+
|
[thioredoxin]-dithiol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.1.8.4.12
- peptide-methionine (R)-S-oxide reductase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-methionyl-[protein] + [thioredoxin]-disulfide + H2O = L-methionyl-(R)- S-oxide-[protein] + [thioredoxin]-dithiol
|
 |
 |
 |
 |
 |
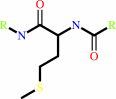 Peptide-L-methionine
Peptide-L-methionine
|
+
|
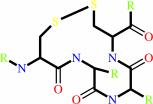 thioredoxin disulfide
thioredoxin disulfide
|
+
|
H(2)O
|
=
|
peptide-L- methionine (R)-S-oxide
|
+
|
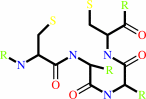 thioredoxin
thioredoxin
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Se(2+); Zn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
47:8577-8589
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
Solution structure and dynamics of the reduced and oxidized forms of the N-terminal domain of PilB from Neisseria meningitidis.
|
|
M.Quinternet,
P.Tsan,
F.Neiers,
C.Beaufils,
S.Boschi-Muller,
M.C.Averlant-Petit,
G.Branlant,
M.T.Cung.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The secreted form of the PilB protein was proposed to be involved in pathogen
survival fighting against the defensive host's oxidative burst. PilB protein is
composed of three domains. The central and the C-terminal domains display
methionine sulfoxide reductase A and B activities, respectively. The N-terminal
domain, which possesses a CXXC motif, was recently shown to regenerate in vitro
the reduced forms of the methionine sulfoxide reductase domains of PilB from
their oxidized forms, as does the thioredoxin 1 from E. coli, via a disulfide
bond exchange. The thioredoxin-like N-terminal domain belongs to the cytochrome
maturation protein structural family, but it possesses a unique additional
segment (99)FLHE (102) localized in a loop. This segment covers one edge of the
active site in the crystal structure of the reduced form of the N-terminal
domain of PilB. We have determined the solution structure and the dynamics of
the N-terminal domain from Neisseria meningitidis, in its reduced and oxidized
forms. The FLHE loop adopts, in both redox states, a well-defined conformation.
Subtle conformational and dynamic changes upon oxidation are highlighted around
the active site, as well as in the FLHE loop. The functional consequences of the
cytochrome maturation protein topology and those of the presence of FLHE loop
are discussed in relation to the enzymatic properties of the N-terminal domain.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.Quinternet,
P.Tsan,
L.Selme-Roussel,
C.Jacob,
S.Boschi-Muller,
G.Branlant,
and
M.T.Cung
(2009).
Formation of the complex between DsbD and PilB N-terminal domains from Neisseria meningitidis necessitates an adaptability of nDsbD.
|
| |
Structure,
17,
1024-1033.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

