 |
PDBsum entry 2ic1

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2ic1
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.13.11.20
- cysteine dioxygenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-cysteine + O2 = 3-sulfino-L-alanine + H+
|
 |
 |
 |
 |
 |
L-cysteine
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 O2
O2
|
=
|
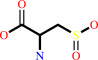 3-sulfino-L-alanine
3-sulfino-L-alanine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe(2+); NADH or NADPH
|
 |
 |
 |
 |
 |
Fe(2+)
|
 NADH
NADH
|
or
|
 NADPH
NADPH
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
282:3391-3402
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
An insight into the mechanism of human cysteine dioxygenase. Key roles of the thioether-bonded tyrosine-cysteine cofactor.
|
|
S.Ye,
X.Wu,
L.Wei,
D.Tang,
P.Sun,
M.Bartlam,
Z.Rao.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Cysteine dioxygenase is a non-heme mononuclear iron metalloenzyme that catalyzes
the oxidation of cysteine to cysteine sulfinic acid with addition of molecular
dioxygen. This irreversible oxidative catabolism of cysteine initiates several
important metabolic pathways related to diverse sulfurate compounds. Cysteine
dioxygenase is therefore very important for maintaining the proper hepatic
concentration of intracellular free cysteine. Mechanisms for mouse and rat
cysteine dioxygenases have recently been reported based on their crystal
structures in the absence of substrates, although there is still a lack of
direct evidence. Here we report the first crystal structure of human cysteine
dioxygenase in complex with its substrate L-cysteine to 2.7A, together with
enzymatic activity and metal content assays of several single point mutants. Our
results provide an insight into a new mechanism of cysteine thiol dioxygenation
catalyzed by cysteine dioxygenase, which is tightly associated with a
thioether-bonded tyrosine-cysteine cofactor involving Tyr-157 and Cys-93. This
cross-linked protein-derived cofactor plays several key roles different from
those in galactose oxidase. This report provides a new potential target for
therapy of diseases related to human cysteine dioxygenase, including
neurodegenerative and autoimmune diseases.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
FIGURE 2. Overall crystal structure of human CDO in complex
with the cysteine substrate. A, a stereo view ribbon
representation with secondary structure assignments. The  -barrel
is made up of two antiparallel -barrel
is made up of two antiparallel  -sheets colored yellow
and brown and a mixed -sheets colored yellow
and brown and a mixed  -sheet colored red.
Helices are colored in cyan, and the ferrous ion is colored
turquoise. The cysteine substrate is shown in ball-and-stick
representation. B, electrostatic potential of the human CDO
crystal structure, calculated using PyMOL (DeLano Scientific,
San Carlos, CA). Negatively charged regions are colored in red,
positively charged regions are blue, and neutral regions are
white. A hole from the surface to the active center is very
clear, through which the substrate cysteine can be seen. C,
superposition of human, mouse, and rat CDO. Human CDO is colored
in red, mouse CDO in green, and rat CDO in blue. Non-conserved
residues are highlighted as sticks. Among these three CDOs, the
central region around the active site is highly conserved both
in primary sequence and in three-dimensional conformation. -sheet colored red.
Helices are colored in cyan, and the ferrous ion is colored
turquoise. The cysteine substrate is shown in ball-and-stick
representation. B, electrostatic potential of the human CDO
crystal structure, calculated using PyMOL (DeLano Scientific,
San Carlos, CA). Negatively charged regions are colored in red,
positively charged regions are blue, and neutral regions are
white. A hole from the surface to the active center is very
clear, through which the substrate cysteine can be seen. C,
superposition of human, mouse, and rat CDO. Human CDO is colored
in red, mouse CDO in green, and rat CDO in blue. Non-conserved
residues are highlighted as sticks. Among these three CDOs, the
central region around the active site is highly conserved both
in primary sequence and in three-dimensional conformation.
|
 |
Figure 7.
FIGURE 7. Catalytic mechanism of human CDO. See the text
for detailed discussion.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2007,
282,
3391-3402)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.H.Stipanuk,
C.R.Simmons,
P.Andrew Karplus,
and
J.E.Dominy
(2011).
Thiol dioxygenases: unique families of cupin proteins.
|
| |
Amino Acids,
41,
91.
|
 |
|
|
|
|
 |
M.H.Stipanuk,
and
I.Ueki
(2011).
Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur.
|
| |
J Inherit Metab Dis,
34,
17-32.
|
 |
|
|
|
|
 |
S.Leitgeb,
G.D.Straganz,
and
B.Nidetzky
(2009).
Functional characterization of an orphan cupin protein from Burkholderia xenovorans reveals a mononuclear nonheme Fe2+-dependent oxygenase that cleaves beta-diketones.
|
| |
FEBS J,
276,
5983-5997.
|
 |
|
|
|
|
 |
T.Kleffmann,
S.A.Jongkees,
G.Fairweather,
S.M.Wilbanks,
and
G.N.Jameson
(2009).
Mass-spectrometric characterization of two posttranslational modifications of cysteine dioxygenase.
|
| |
J Biol Inorg Chem,
14,
913-921.
|
 |
|
|
|
|
 |
C.R.Simmons,
K.Krishnamoorthy,
S.L.Granett,
D.J.Schuller,
J.E.Dominy,
T.P.Begley,
M.H.Stipanuk,
and
P.A.Karplus
(2008).
A putative Fe2+-bound persulfenate intermediate in cysteine dioxygenase.
|
| |
Biochemistry,
47,
11390-11392.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.E.Dominy,
J.Hwang,
S.Guo,
L.L.Hirschberger,
S.Zhang,
and
M.H.Stipanuk
(2008).
Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity.
|
| |
J Biol Chem,
283,
12188-12201.
|
 |
|
|
|
|
 |
M.H.Stipanuk,
J.E.Dominy,
I.Ueki,
and
L.L.Hirschberger
(2008).
Measurement of Cysteine Dioxygenase Activity and Protein Abundance.
|
| |
Curr Protoc Toxicol,
38,
6.15.1.
|
 |
|
|
|
|
 |
T.D.Bugg,
and
S.Ramaswamy
(2008).
Non-heme iron-dependent dioxygenases: unravelling catalytic mechanisms for complex enzymatic oxidations.
|
| |
Curr Opin Chem Biol,
12,
134-140.
|
 |
|
|
|
|
 |
Y.K.Lee,
M.M.Whittaker,
and
J.W.Whittaker
(2008).
The electronic structure of the Cys-Tyr(*) free radical in galactose oxidase determined by EPR spectroscopy.
|
| |
Biochemistry,
47,
6637-6649.
|
 |
|
|
|
|
 |
G.N.Phillips,
B.G.Fox,
J.L.Markley,
B.F.Volkman,
E.Bae,
E.Bitto,
C.A.Bingman,
R.O.Frederick,
J.G.McCoy,
B.L.Lytle,
B.S.Pierce,
J.Song,
and
S.N.Twigger
(2007).
Structures of proteins of biomedical interest from the Center for Eukaryotic Structural Genomics.
|
| |
J Struct Funct Genomics,
8,
73-84.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

