 |
PDBsum entry 2htb

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.5.1.3.15
- glucose-6-phosphate 1-epimerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
alpha-D-glucose 6-phosphate = beta-D-glucose 6-phosphate
|
 |
 |
 |
 |
 |
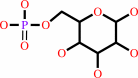 alpha-D-glucose 6-phosphate
alpha-D-glucose 6-phosphate
|
=
|
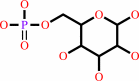 beta-D-glucose 6-phosphate
beta-D-glucose 6-phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Acta Crystallogr D Biol Crystallogr
63:197-205
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of the putative mutarotase YeaD from Salmonella typhimurium: structural comparison with galactose mutarotases.
|
|
S.Chittori,
D.K.Simanshu,
H.S.Savithri,
M.R.Murthy.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Salmonella typhimurium YeaD (stYeaD), annotated as a putative aldose
1-epimerase, has a very low sequence identity to other well characterized
mutarotases. Sequence analysis suggested that the catalytic residues and a few
of the substrate-binding residues of galactose mutarotases (GalMs) are conserved
in stYeaD. Determination of the crystal structure of stYeaD in an orthorhombic
form at 1.9 A resolution and in a monoclinic form at 2.5 A resolution revealed
this protein to adopt the beta-sandwich fold similar to GalMs. Structural
comparison of stYeaD with GalMs has permitted the identification of residues
involved in catalysis and substrate binding. In spite of the similar fold and
conservation of catalytic residues, minor but significant differences were
observed in the substrate-binding pocket. These analyses pointed out the
possible role of Arg74 and Arg99, found only in YeaD-like proteins, in ligand
anchoring and suggested that the specificity of stYeaD may be distinct from
those of GalMs.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |
Figure 3.
Figure 3 Superposition of the active-site residues of (a)
stYeaD and lacGalM and (b) stYeaD and YMR099cp. Colour code:
green, lacGalM; wheat, stYeaD; pink; YMR099cp.
|
 |
|
|

|
| |
The above figure is
reprinted
by permission from the IUCr:
Acta Crystallogr D Biol Crystallogr
(2007,
63,
197-205)
copyright 2007.
|
|
| |
Figure was
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

