 |
PDBsum entry 1zrs

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.4.17.13
- muramoyltetrapeptide carboxypeptidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N-acetyl-D-glucosaminyl-N-acetylmuramoyl-L-alanyl-meso-2,6- diaminoheptanedioyl-D-alanine + H2O = N-acetyl-D-glucosaminyl-N- acetylmuramoyl-L-alanyl-meso-2,6-diaminoheptanedioate + D-alanine
|
 |
 |
 |
 |
 |
N-acetyl-D-glucosaminyl-N-acetylmuramoyl-L-alanyl-meso-2,6- diaminoheptanedioyl-D-alanine
|
+
|
H2O
|
=
|
N-acetyl-D-glucosaminyl-N- acetylmuramoyl-L-alanyl-meso-2,6-diaminoheptanedioate
|
+
|
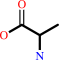 D-alanine
D-alanine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
280:40802-40812
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Pseudomonas aeruginosa LD-carboxypeptidase, a serine peptidase with a Ser-His-Glu triad and a nucleophilic elbow.
|
|
H.J.Korza,
M.Bochtler.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
LD-Carboxypeptidases (EC 3.4.17.13) are named for their ability to cleave amide
bonds between l- and d-amino acids, which occur naturally in bacterial
peptidoglycan. They are specific for the link between meso-diaminopimelic acid
and d-alanine and therefore degrade GlcNAc-MurNAc tetrapeptides to the
corresponding tripeptides. As only the tripeptides can be reused as
peptidoglycan building blocks, ld-carboxypeptidases are thought to play a role
in peptidoglycan recycling. Despite the pharmaceutical interest in peptidoglycan
biosynthesis, the fold and catalytic type of ld-carboxypeptidases are unknown.
Here, we show that a previously uncharacterized open reading frame in
Pseudomonas aeruginosa has ld-carboxypeptidase activity and present the crystal
structure of this enzyme. The structure shows that the enzyme consists of an
N-terminal beta-sheet and a C-terminal beta-barrel domain. At the interface of
the two domains, Ser(115) adopts a highly strained conformation in the context
of a strand-turn-helix motif that is similar to the "nucleophilic
elbow" in alphabeta-hydrolases. Ser(115) is hydrogen-bonded to a histidine
residue, which is oriented by a glutamate residue. All three residues, which
occur in the order Ser-Glu-His in the amino acid sequence, are strictly
conserved in naturally occurring ld-carboxypeptidases and cannot be mutated to
alanines without loss of activity. We conclude that ld-carboxypeptidases are
serine peptidases with Ser-His-Glu catalytic triads.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
FIGURE 1. Schematic representation of the disaccharide tri-
and tetrapeptide fragments resulting from digestion of P. putida
peptidoglycan with lysozyme. The calculated and experimentally
measured monoisotopic masses of the most abundant species are
indicated. Stereochemical information was taken from Refs. 8 and
15, and the glycosidic linkage was placed between C-1 of GlcNAc
and C-4 of MurNAc and not between C-1 of MurNAc and C-4 of
GlcNAc. This choice was based on the known preference of
lysozyme for the glycosidic link that connects C-1 of MurNAc to
C-4 of GlcNAc (16). S and P stand for substrate and product of
the enzymatic reaction.
|
 |
Figure 9.
FIGURE 9. Comparison of the nucleophilic elbows in
LD-carboxypeptidase (A and B) and the lipase from G. candidum as
a representative of   -hydrolases (C and D). A
and C show C- -hydrolases (C and D). A
and C show C-  traces in
monorepresentation, and B and D show all atom representations in
stereo. The solid green lines show hydrogen bonds that match the
pattern for 3[10]-helices, and dashed green lines show hydrogen
bonds in regular traces in
monorepresentation, and B and D show all atom representations in
stereo. The solid green lines show hydrogen bonds that match the
pattern for 3[10]-helices, and dashed green lines show hydrogen
bonds in regular  -helices. The figure
was made with the MolScript program (54). -helices. The figure
was made with the MolScript program (54).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2005,
280,
40802-40812)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
N.Nikoh,
and
A.Nakabachi
(2009).
Aphids acquired symbiotic genes via lateral gene transfer.
|
| |
BMC Biol,
7,
12.
|
 |
|
|
|
|
 |
P.Rossi,
J.M.Aramini,
R.Xiao,
C.X.Chen,
C.Nwosu,
L.A.Owens,
M.Maglaqui,
R.Nair,
M.Fischer,
T.B.Acton,
B.Honig,
B.Rost,
and
G.T.Montelione
(2009).
Structural elucidation of the Cys-His-Glu-Asn proteolytic relay in the secreted CHAP domain enzyme from the human pathogen Staphylococcus saprophyticus.
|
| |
Proteins,
74,
515-519.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.J.Patti,
J.Chen,
J.Schaefer,
and
M.L.Gross
(2008).
Characterization of structural variations in the peptidoglycan of vancomycin-susceptible Enterococcus faecium: understanding glycopeptide-antibiotic binding sites using mass spectrometry.
|
| |
J Am Soc Mass Spectrom,
19,
1467-1475.
|
 |
|
|
|
|
 |
J.T.Park,
and
T.Uehara
(2008).
How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan).
|
| |
Microbiol Mol Biol Rev,
72,
211.
|
 |
|
|
|
|
 |
O.D.Ekici,
M.Paetzel,
and
R.E.Dalbey
(2008).
Unconventional serine proteases: variations on the catalytic Ser/His/Asp triad configuration.
|
| |
Protein Sci,
17,
2023-2037.
|
 |
|
|
|
|
 |
W.Vollmer,
B.Joris,
P.Charlier,
and
S.Foster
(2008).
Bacterial peptidoglycan (murein) hydrolases.
|
| |
FEMS Microbiol Rev,
32,
259-286.
|
 |
|
|
|
|
 |
M.Firczuk,
and
M.Bochtler
(2007).
Folds and activities of peptidoglycan amidases.
|
| |
FEMS Microbiol Rev,
31,
676-691.
|
 |
|
|
|
|
 |
P.Courtin,
G.Miranda,
A.Guillot,
F.Wessner,
C.Mézange,
E.Domakova,
S.Kulakauskas,
and
M.P.Chapot-Chartier
(2006).
Peptidoglycan structure analysis of Lactococcus lactis reveals the presence of an L,D-carboxypeptidase involved in peptidoglycan maturation.
|
| |
J Bacteriol,
188,
5293-5298.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

