 |
PDBsum entry 1y2c

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Catalytic domain of human phosphodiesterase 4d in complex with 3,5- dimethyl-1-phenyl-1h-pyrazole-4-carboxylic acid ethyl ester
|
|
Structure:
|
 |
Camp-specific 3',5'-cyclic phosphodiesterase 4d. Chain: a, b. Fragment: catalytic domain of human phosphodiesterase 4d. Synonym: dpde3, pde43. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: pde4d. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Biol. unit:
|
 |
Dimer (from
)
|
|
Resolution:
|
 |
|
1.67Å
|
R-factor:
|
0.177
|
R-free:
|
0.190
|
|
|
Authors:
|
 |
G.L.Card,L.Blasdel,B.P.England,C.Zhang,Y.Suzuki,S.Gillette,D.Fong, P.N.Ibrahim,D.R.Artis,G.Bollag,M.V.Milburn,S.-H.Kim,J.Schlessinger, K.Y.J.Zhang
|
Key ref:

|
 |
G.L.Card
et al.
(2005).
A family of phosphodiesterase inhibitors discovered by cocrystallography and scaffold-based drug design.
Nat Biotechnol,
23,
201-207.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
22-Nov-04
|
Release date:
|
01-Mar-05
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q08499
(PDE4D_HUMAN) -
3',5'-cyclic-AMP phosphodiesterase 4D from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
809 a.a.
326 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.4.53
- 3',5'-cyclic-AMP phosphodiesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
3',5'-cyclic AMP + H2O = AMP + H+
|
 |
 |
 |
 |
 |
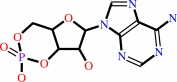 3',5'-cyclic AMP
3',5'-cyclic AMP
|
+
|
H2O
|
=
|
 AMP
AMP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nat Biotechnol
23:201-207
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
A family of phosphodiesterase inhibitors discovered by cocrystallography and scaffold-based drug design.
|
|
G.L.Card,
L.Blasdel,
B.P.England,
C.Zhang,
Y.Suzuki,
S.Gillette,
D.Fong,
P.N.Ibrahim,
D.R.Artis,
G.Bollag,
M.V.Milburn,
S.H.Kim,
J.Schlessinger,
K.Y.Zhang.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Cyclic nucleotide phosphodiesterases (PDEs) comprise a large family of enzymes
that regulate a variety of cellular processes. We describe a family of potent
PDE4 inhibitors discovered using an efficient method for scaffold-based drug
design. This method involves an iterative approach starting with low-affinity
screening of compounds followed by high-throughput cocrystallography to reveal
the molecular basis underlying the activity of the newly identified compounds.
Through detailed structural analysis of the interaction of the initially
discovered pyrazole carboxylic ester scaffold with PDE4D using X-ray
crystallography, we identified three sites of chemical substitution and designed
small selective libraries of scaffold derivatives with modifications at these
sites. A 4,000-fold increase in the potency of this PDE4 inhibitor was achieved
after only two rounds of chemical synthesis and the structural analysis of seven
pyrazole derivatives bound to PDE4B or PDE4D, revealing the robustness of this
approach for identifying new inhibitors that can be further developed into drug
candidates.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. Crystal structure of the pyrazole scaffold and its
derivatives in complex with PDE4B or PDE4D. (a) Crystal
structure of 3,5-dimethyl-1H-pyrazole-4-carboxylic acid ethyl
ester (pyrazole no. 2) bound to PDE4D, showing the pyrazole ring
sandwiched in the hydrophobic clamp formed by F372 and I336. The
conserved H-bond, seen in all pyrazole derivative cocrystal
structures, between the NE2 atom of the invariant glutamine and
the carboxylate group, is shown. (b) The crystal structure of
3,5-dimethyl-1-phenyl-1H-pyrazole-4-carboxylic acid ethyl ester
(pyrazole no. 8) bound to PDE4D, showing the same interactions
as its parent compound, and thus validating the dimethyl
pyrazole as a scaffold. The dimethyl pyrazole is sandwiched by
F372 and I336 and the carbonyl oxygen forms an H-bond with Q369.
The ethoxy group is tucked into the Q1 pocket. (c) Crystal
structure of
3,5-dimethyl-1-(3-nitro-phenyl)-1H-pyrazole-4-carboxylic acid
ethyl ester (pyrazole no. 21) bound to PDE4B and PDE4D. The
carbon atoms of pyrazole no. 21 bound to PDE4B and PDE4D are
shown in green and yellow respectively. The NO[2] group at the
meta-position of the phenyl ring formed H-bonds with T345, D392
in PDE4B and the two water molecules coordinating Zn2+ (omitted
for clarity). (d) Crystal structure of
1-(2-chloro-phenyl)-3,5-dimethyl-1H-pyrazole-4-carboxylic acid
ethyl ester (pyrazole no. 20) bound to PDE4B. The
Cl-substitution at the ortho-position of the phenyl ring makes
several hydrophobic contacts with residues M347, L393 and F446.
(e) Crystal structure of
1-(4-amino-phenyl)-3,5-dimethyl-1H-pyrazole-4-carboxylic acid
ethyl ester (pyrazole no. 19) bound to PDE4D. The amine group
forms three H-bonds with three water molecules, two of which are
coordinated to Mg2+. However, this amine nitrogen is also in
close proximity to the carbon atom in M273 which results in
unfavorable interactions. (f) Crystal structure of
1-(4-methoxy-phenyl)-3,5-dimethyl-1H-pyrazole-4-carboxylic acid
ethyl ester (pyrazole no. 17) bound to PDE4D. The methoxy-phenyl
group rotated 180° to point away from the di-metal ions to avoid
the repulsive interactions between the methoxy group and the
di-metal ions.
|
 |
Figure 3.
Figure 3. Pyrazole scaffold bound to PDE4B and PDE4D and the
discovery of potent pyrazole inhibitors for PDE4 in three steps.
Superposition of six different pyrazoles (nos. 2, 8, 17, 19,
20 and 21) in seven cocrystal structures with PDE4B and/or PDE4D
revealed the consistent binding mode of the scaffold moiety
(panels a -c). For clarity, only several side chains for one
PDE4B cocrystal structure are shown. The three pockets in the
active site are highlighted on the solvent accessible surface:
the metal binding pocket (M) in blue, purine-selective glutamine
and hydrophobic clamp pocket (Q) in red (which is further
divided into Q[1], Q[2] sub-pockets) and solvent-filled side
pocket (S) in green. The discovery of potent pyrazole inhibitors
for PDE4 in three steps is illustrated in panels d -f. (a) A
view looking down into the active site. The pyrazole carboxylate
scaffold fits into the narrow passage formed by the hydrophobic
clamp. (b) A view looking away from the S pocket. The pyrazole
carboxylate scaffold forms an H-bond with the invariant Q443^4B.
(c) A view looking towards the S pocket. The ethoxy group
occupies the Q[1]-pocket. The scaffold that the six different
pyrazoles share is marked by a dashed oval. (d) Scaffold
discovery. The scaffold candidate,
3,5-dimethyl-1H-pyrazole-4-carboxylic acid ethyl ester (pyrazole
no. 2), is a weak PDE4D inhibitor with IC[50] of 82  M.
(e) Scaffold validation. The derivative of the scaffold,
3,5-dimethyl-1-phenyl-1H-pyrazole-4-carboxylic acid ethyl ester
(pyrazole no. 8) has significantly increased potency towards
PDE4D with IC[50] of 0.27 M.
(e) Scaffold validation. The derivative of the scaffold,
3,5-dimethyl-1-phenyl-1H-pyrazole-4-carboxylic acid ethyl ester
(pyrazole no. 8) has significantly increased potency towards
PDE4D with IC[50] of 0.27  M.
(f) Chemical optimization. The validated scaffold was optimized
into a potent PDE4D inhibitor,
3,5-dimethyl-1-(3-nitro-phenyl)-1H-pyrazole-4-carboxylic acid
ethyl ester (pyrazole no. 21), with IC[50] of 0.021 M.
(f) Chemical optimization. The validated scaffold was optimized
into a potent PDE4D inhibitor,
3,5-dimethyl-1-(3-nitro-phenyl)-1H-pyrazole-4-carboxylic acid
ethyl ester (pyrazole no. 21), with IC[50] of 0.021  M.
A 4,000-fold increase in potency was achieved in two rounds of
chemical synthesis. Compounds are represented by solid surface
colored by atomic types. The active site is represented by the
blue mesh. The PDE4D is represented by cartoons where helices
are shown as cylinders and loops are shown as tubes. M.
A 4,000-fold increase in potency was achieved in two rounds of
chemical synthesis. Compounds are represented by solid surface
colored by atomic types. The active site is represented by the
blue mesh. The PDE4D is represented by cartoons where helices
are shown as cylinders and loops are shown as tubes.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nat Biotechnol
(2005,
23,
201-207)
copyright 2005.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
L.Alvarez Thon,
C.Bustos,
F.Diaz-Marín,
M.T.Garland,
and
R.Baggio
(2013).
3,5-Dimethyl-4-[(E)-(2-nitrophenyl)diazenyl]-1-(2,3,4,5,6-pentafluorophenyl)-1H-pyrazole.
|
| |
Acta Crystallogr C,
69,
101-104.
|
 |
|
|
|
|
 |
C.Bustos,
M.Pérez-Cerda,
L.Alvarez-Thon,
E.Barrales-Salcedo,
and
M.T.Garland
(2012).
(E)-3,5-Dimethyl-1-p-tolyl-4-(p-tolyl-diazen-yl)-1H-pyrazole.
|
| |
Acta Crystallogr Sect E Struct Rep Online,
68,
o353-o354.
|
 |
|
|
|
|
 |
G.Bollag,
J.Tsai,
J.Zhang,
C.Zhang,
P.Ibrahim,
K.Nolop,
and
P.Hirth
(2012).
Vemurafenib: the first drug approved for BRAF-mutant cancer.
|
| |
Nat Rev Drug Discov,
11,
873-886.
|
 |
|
|
|
|
 |
K.Ohno,
T.Mitsui,
Y.Tanida,
A.Matsuura,
H.Fujitani,
T.Niimi,
and
M.Orita
(2011).
Docking study and binding free energy calculation of poly (ADP-ribose) polymerase inhibitors.
|
| |
J Mol Model,
17,
383-389.
|
 |
|
|
|
|
 |
R.E.Hubbard
(2011).
Structure-based drug discovery and protein targets in the CNS.
|
| |
Neuropharmacology,
60,
7.
|
 |
|
|
|
|
 |
M.A.Alaamery,
A.R.Wyman,
F.D.Ivey,
C.Allain,
D.Demirbas,
L.Wang,
O.Ceyhan,
and
C.S.Hoffman
(2010).
New classes of PDE7 inhibitors identified by a fission yeast-based HTS.
|
| |
J Biomol Screen,
15,
359-367.
|
 |
|
|
|
|
 |
D.R.Artis,
J.J.Lin,
C.Zhang,
W.Wang,
U.Mehra,
M.Perreault,
D.Erbe,
H.I.Krupka,
B.P.England,
J.Arnold,
A.N.Plotnikov,
A.Marimuthu,
H.Nguyen,
S.Will,
M.Signaevsky,
J.Kral,
J.Cantwell,
C.Settachatgull,
D.S.Yan,
D.Fong,
A.Oh,
S.Shi,
P.Womack,
B.Powell,
G.Habets,
B.L.West,
K.Y.Zhang,
M.V.Milburn,
G.P.Vlasuk,
K.P.Hirth,
K.Nolop,
G.Bollag,
P.N.Ibrahim,
and
J.F.Tobin
(2009).
Scaffold-based discovery of indeglitazar, a PPAR pan-active anti-diabetic agent.
|
| |
Proc Natl Acad Sci U S A,
106,
262-267.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
G.E.de Kloe,
D.Bailey,
R.Leurs,
and
I.J.de Esch
(2009).
Transforming fragments into candidates: small becomes big in medicinal chemistry.
|
| |
Drug Discov Today,
14,
630-646.
|
 |
|
|
|
|
 |
I.Miyazaki,
S.Simizu,
K.Ishida,
and
H.Osada
(2009).
On-chip fragment-based approach for discovery of high-affinity bivalent inhibitors.
|
| |
Chembiochem,
10,
838-843.
|
 |
|
|
|
|
 |
K.Yang,
C.H.Trepanier,
H.Li,
M.A.Beazely,
E.A.Lerner,
M.F.Jackson,
and
J.F.MacDonald
(2009).
Vasoactive intestinal peptide acts via multiple signal pathways to regulate hippocampal NMDA receptors and synaptic transmission.
|
| |
Hippocampus,
19,
779-789.
|
 |
|
|
|
|
 |
M.Orita,
K.Ohno,
and
T.Niimi
(2009).
Two 'Golden Ratio' indices in fragment-based drug discovery.
|
| |
Drug Discov Today,
14,
321-328.
|
 |
|
|
|
|
 |
Y.Chen,
and
B.K.Shoichet
(2009).
Molecular docking and ligand specificity in fragment-based inhibitor discovery.
|
| |
Nat Chem Biol,
5,
358-364.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.Chen,
M.Misra,
L.Sower,
J.W.Peterson,
G.E.Kellogg,
and
C.H.Schein
(2008).
Novel inhibitors of anthrax edema factor.
|
| |
Bioorg Med Chem,
16,
7225-7233.
|
 |
|
|
|
|
 |
J.Tsai,
J.T.Lee,
W.Wang,
J.Zhang,
H.Cho,
S.Mamo,
R.Bremer,
S.Gillette,
J.Kong,
N.K.Haass,
K.Sproesser,
L.Li,
K.S.Smalley,
D.Fong,
Y.L.Zhu,
A.Marimuthu,
H.Nguyen,
B.Lam,
J.Liu,
I.Cheung,
J.Rice,
Y.Suzuki,
C.Luu,
C.Settachatgul,
R.Shellooe,
J.Cantwell,
S.H.Kim,
J.Schlessinger,
K.Y.Zhang,
B.L.West,
B.Powell,
G.Habets,
C.Zhang,
P.N.Ibrahim,
P.Hirth,
D.R.Artis,
M.Herlyn,
and
G.Bollag
(2008).
Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity.
|
| |
Proc Natl Acad Sci U S A,
105,
3041-3046.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.E.Hubbard
(2008).
Fragment approaches in structure-based drug discovery.
|
| |
J Synchrotron Radiat,
15,
227-230.
|
 |
|
|
|
|
 |
T.Hesterkamp,
and
M.Whittaker
(2008).
Fragment-based activity space: smaller is better.
|
| |
Curr Opin Chem Biol,
12,
260-268.
|
 |
|
|
|
|
 |
E.Evensen,
D.Joseph-McCarthy,
G.A.Weiss,
S.L.Schreiber,
and
M.Karplus
(2007).
Ligand design by a combinatorial approach based on modeling and experiment: application to HLA-DR4.
|
| |
J Comput Aided Mol Des,
21,
395-418.
|
 |
|
|
|
|
 |
G.Siegal,
E.Ab,
and
J.Schultz
(2007).
Integration of fragment screening and library design.
|
| |
Drug Discov Today,
12,
1032-1039.
|
 |
|
|
|
|
 |
H.Jhoti,
A.Cleasby,
M.Verdonk,
and
G.Williams
(2007).
Fragment-based screening using X-ray crystallography and NMR spectroscopy.
|
| |
Curr Opin Chem Biol,
11,
485-493.
|
 |
|
|
|
|
 |
N.Marino,
and
M.Zollo
(2007).
Understanding h-prune biology in the fight against cancer.
|
| |
Clin Exp Metastasis,
24,
637-645.
|
 |
|
|
|
|
 |
P.J.Hajduk,
and
J.Greer
(2007).
A decade of fragment-based drug design: strategic advances and lessons learned.
|
| |
Nat Rev Drug Discov,
6,
211-219.
|
 |
|
|
|
|
 |
A.Ghavami,
W.D.Hirst,
and
T.J.Novak
(2006).
Selective phosphodiesterase (PDE)-4 inhibitors: a novel approach to treating memory deficit?
|
| |
Drugs R D,
7,
63-71.
|
 |
|
|
|
|
 |
D.A.Erlanson
(2006).
Fragment-based lead discovery: a chemical update.
|
| |
Curr Opin Biotechnol,
17,
643-652.
|
 |
|
|
|
|
 |
G.M.Keseru,
and
G.M.Makara
(2006).
Hit discovery and hit-to-lead approaches.
|
| |
Drug Discov Today,
11,
741-748.
|
 |
|
|
|
|
 |
I.Collins,
and
P.Workman
(2006).
New approaches to molecular cancer therapeutics.
|
| |
Nat Chem Biol,
2,
689-700.
|
 |
|
|
|
|
 |
K.Babaoglu,
and
B.K.Shoichet
(2006).
Deconstructing fragment-based inhibitor discovery.
|
| |
Nat Chem Biol,
2,
720-723.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Q.Huai,
Y.Sun,
H.Wang,
D.Macdonald,
R.Aspiotis,
H.Robinson,
Z.Huang,
and
H.Ke
(2006).
Enantiomer discrimination illustrated by the high resolution crystal structures of type 4 phosphodiesterase.
|
| |
J Med Chem,
49,
1867-1873.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
W.Wang,
A.Marimuthu,
J.Tsai,
A.Kumar,
H.I.Krupka,
C.Zhang,
B.Powell,
Y.Suzuki,
H.Nguyen,
M.Tabrizizad,
C.Luu,
and
B.L.West
(2006).
Structural characterization of autoinhibited c-Met kinase produced by coexpression in bacteria with phosphatase.
|
| |
Proc Natl Acad Sci U S A,
103,
3563-3568.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
X.Barril,
and
R.Soliva
(2006).
Molecular modelling.
|
| |
Mol Biosyst,
2,
660-681.
|
 |
|
|
|
|
 |
E.R.Zartler,
and
M.J.Shapiro
(2005).
Fragonomics: fragment-based drug discovery.
|
| |
Curr Opin Chem Biol,
9,
366-370.
|
 |
|
|
|
|
 |
E.S.Kawasaki,
and
A.Player
(2005).
Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer.
|
| |
Nanomedicine,
1,
101-109.
|
 |
|
|
|
|
 |
H.Jhoti
(2005).
A new school for screening.
|
| |
Nat Biotechnol,
23,
184-186.
|
 |
|
|
|
|
 |
K.Y.Zhang,
P.N.Ibrahim,
S.Gillette,
and
G.Bollag
(2005).
Phosphodiesterase-4 as a potential drug target.
|
| |
Expert Opin Ther Targets,
9,
1283-1305.
|
 |
|
|
|
|
 |
L.W.Tari,
M.Rosenberg,
and
A.B.Schryvers
(2005).
Structural proteomics in drug discovery.
|
| |
Expert Rev Proteomics,
2,
511-519.
|
 |
|
|
|
|
 |
M.D.Houslay,
P.Schafer,
and
K.Y.Zhang
(2005).
Keynote review: phosphodiesterase-4 as a therapeutic target.
|
| |
Drug Discov Today,
10,
1503-1519.
|
 |
|
|
|
|
 |
S.M.Greenberg,
and
J.Rosand
(2005).
The phosphodiesterase puzzlebox: PDE4D and stroke.
|
| |
Ann Neurol,
58,
345-346.
|
 |
|
|
|
|
 |
S.P.Williams,
L.F.Kuyper,
and
K.H.Pearce
(2005).
Recent applications of protein crystallography and structure-guided drug design.
|
| |
Curr Opin Chem Biol,
9,
371-380.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

