 |
PDBsum entry 1w3v

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.21.3.1
- isopenicillin-N synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Penicillin N and Deacetoxycephalosporin C Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N-[(5S)-5-amino-5-carboxypentanoyl]-L-cysteinyl-D-valine + O2 = isopenicillin N + 2 H2O
|
 |
 |
 |
 |
 |
N-[(5S)-5-amino-5-carboxypentanoyl]-L-cysteinyl-D-valine
|
+
|
 O2
O2
|
=
|
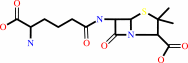 isopenicillin N
isopenicillin N
|
+
|
2
×
H2O
Bound ligand (Het Group name = )
matches with 48.48% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Chembiochem
7:351-358
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Unexpected oxidation of a depsipeptide substrate analogue in crystalline isopenicillin N synthase.
|
|
A.Daruzzaman,
I.J.Clifton,
R.M.Adlington,
J.E.Baldwin,
P.J.Rutledge.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Isopenicillin N synthase (IPNS) is a non-heme iron(ii)-dependent oxidase that is
central to penicillin biosynthesis. Herein, we report mechanistic studies of the
IPNS reaction in the crystalline state, using the substrate analogue
delta-(L-alpha-aminoadipoyl)-(3R)-methyl-L-cysteine D-alpha-hydroxyisovaleryl
ester (AmCOV) to probe the early stages of the catalytic cycle. The X-ray
crystal structure of the anaerobic IPNS:Fe(II):AmCOV complex was solved to 1.40
A resolution, and it reveals several subtle differences in the active site
relative to the complex of the enzyme with its natural substrate. The
crystalline IPNS:Fe(II):AmCOV complex was then exposed to oxygen gas at high
pressure; this brought about reaction to give what appears to be a
hydroxymethyl/ene-thiol product. A mechanism for this reaction is proposed.
These results offer further insight into the delicate interplay of steric and
electronic effects in the IPNS active site and the mechanistic intricacies of
this remarkable enzyme.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
V.J.Dungan,
Y.Ortin,
H.Mueller-Bunz,
and
P.J.Rutledge
(2010).
Design and synthesis of a tetradentate '3-amine-1-carboxylate' ligand to mimic the metal binding environment at the non-heme iron(II) oxidase active site.
|
| |
Org Biomol Chem,
8,
1666-1673.
|
 |
|
|
|
|
 |
W.Ge,
I.J.Clifton,
A.R.Howard-Jones,
J.E.Stok,
R.M.Adlington,
J.E.Baldwin,
and
P.J.Rutledge
(2009).
Structural studies on the reaction of isopenicillin N synthase with a sterically demanding depsipeptide substrate analogue.
|
| |
Chembiochem,
10,
2025-2031.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
P.C.Bruijnincx,
G.van Koten,
and
R.J.Klein Gebbink
(2008).
Mononuclear non-heme iron enzymes with the 2-His-1-carboxylate facial triad: recent developments in enzymology and modeling studies.
|
| |
Chem Soc Rev,
37,
2716-2744.
|
 |
|
|
|
|
 |
A.C.Stewart,
I.J.Clifton,
R.M.Adlington,
J.E.Baldwin,
and
P.J.Rutledge
(2007).
A cyclobutanone analogue mimics penicillin in binding to isopenicillin N synthase.
|
| |
Chembiochem,
8,
2003-2007.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

