 |
PDBsum entry 1w3u

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.6.1.52
- phosphoserine transaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
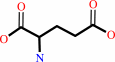
O-phospho-L-serine + 2-oxoglutarate = 3-phosphooxypyruvate + L-glutamate
|
|
2.
|
4-(phosphooxy)-L-threonine + 2-oxoglutarate = (R)-3-hydroxy-2-oxo-4- phosphooxybutanoate + L-glutamate
|
|
 |
 |
 |
 |
 |
O-phospho-L-serine
Bound ligand (Het Group name = )
matches with 45.45% similarity
|
+
|
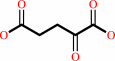 2-oxoglutarate
2-oxoglutarate
|
=
|
3-phosphooxypyruvate
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
4-(phosphooxy)-L-threonine
Bound ligand (Het Group name = )
matches with 45.45% similarity
|
+
|
 2-oxoglutarate
2-oxoglutarate
|
=
|
(R)-3-hydroxy-2-oxo-4- phosphooxybutanoate
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Protein Sci
14:97
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Enzyme adaptation to alkaline pH: atomic resolution (1.08 A) structure of phosphoserine aminotransferase from Bacillus alcalophilus.
|
|
A.P.Dubnovitsky,
E.G.Kapetaniou,
A.C.Papageorgiou.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of the vitamin B(6)-dependent enzyme phosphoserine
aminotransferase from the obligatory alkaliphile Bacillus alcalophilus has been
determined at 1.08 A resolution. The model was refined to an R-factor of 11.7%
(R(free) = 13.9%). The enzyme displays a narrow pH optimum of enzymatic activity
at pH 9.0. The final structure was compared to the previously reported structure
of the mesophilic phosphoserine aminotransferase from Escherichia coli and to
that of phosphoserine aminotransferase from a facultative alkaliphile, Bacillus
circulans subsp. alkalophilus. All three enzymes are homodimers with each
monomer comprising a two-domain architecture. Despite the high structural
similarity, the alkaliphilic representatives possess a set of distinctive
structural features. Two residues directly interacting with
pyridoxal-5'-phosphate are replaced, and an additional hydrogen bond to the O3'
atom of the cofactor is present in alkaliphilic phosphoserine aminotransferases.
The number of hydrogen bonds and hydrophobic interactions at the dimer interface
is increased. Hydrophobic interactions between the two domains in the monomers
are enhanced. Moreover, the number of negatively charged amino acid residues
increases on the solvent-accessible molecular surface and fewer hydrophobic
residues are exposed to the solvent. Further, the total amount of ion pairs and
ion networks is significantly reduced in the Bacillus enzymes, while the total
number of hydrogen bonds is increased. The mesophilic enzyme from Escherichia
coli contains two additional beta-strands in a surface loop with a third
beta-strand being shorter in the structure. The identified structural features
are proposed to be possible factors implicated in the alkaline adaptation of
phosphoserine aminotransferase.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Ribbon representation of the BALC PSAT dimer. The
two monomers are depicted in yellow and in blue, respectively.
Ligands included in the final model (PLP, PEG, HEPES, glycerol)
are shown in ball-and-sticks. Mg2+ ions are shown as gray
spheres and Cl- ions as pink spheres. (A) View along the twofold
noncrystallographic axis. The active site clefts are shown with
arrows. (B) BALC PSAT dimer after 90° rotation. The twofold axis
in this orientation is vertical and lying within the plane of
the figure. The figure was produced using MOLSCRIPT (Kraulis
1991) and Raster3D (Merritt and Murphy 1994).
|
 |
Figure 5.
Figure 5. Definition of torsion angle for the internal
aldimine bond and atom names in pyridoxal-5'-phosphate. The
figure was produced using ISIS/Draw (MDL, Inc.).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the Protein Society:
Protein Sci
(2005,
14,
97-0)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Y.Zhao,
Y.Zhang,
Y.Cao,
J.Qi,
L.Mao,
Y.Xue,
F.Gao,
H.Peng,
X.Wang,
G.F.Gao,
and
Y.Ma
(2011).
Structural analysis of alkaline β-mannanase from alkaliphilic Bacillus sp. N16-5: implications for adaptation to alkaline conditions.
|
| |
PLoS One,
6,
e14608.
|
 |
|
|
|
|
 |
V.Mishra,
V.Ali,
T.Nozaki,
and
V.Bhakuni
(2010).
Entamoeba histolytica Phosphoserine aminotransferase (EhPSAT): insights into the structure-function relationship.
|
| |
BMC Res Notes,
3,
52.
|
 |
|
|
|
|
 |
C.J.Liao,
K.H.Chin,
C.H.Lin,
P.S.Tsai,
P.C.Lyu,
C.C.Young,
A.H.Wang,
and
S.H.Chou
(2008).
Crystal structure of DFA0005 complexed with alpha-ketoglutarate: a novel member of the ICL/PEPM superfamily from alkali-tolerant Deinococcus ficus.
|
| |
Proteins,
73,
362-371.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.Nishibori,
T.Nakamura,
M.Arimoto,
S.Aoyagi,
H.Ago,
M.Miyano,
T.Ebisuzaki,
and
M.Sakata
(2008).
Application of maximum-entropy maps in the accurate refinement of a putative acylphosphatase using 1.3 A X-ray diffraction data.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
237-247.
|
 |
|
|
|
|
 |
A.Quesada,
M.I.Guijo,
F.Merchán,
B.Blázquez,
M.I.Igeño,
and
R.Blasco
(2007).
Essential role of cytochrome bd-related oxidase in cyanide resistance of Pseudomonas pseudoalcaligenes CECT5344.
|
| |
Appl Environ Microbiol,
73,
5118-5124.
|
 |
|
|
|
|
 |
L.Redecke,
M.A.Brehm,
and
R.Bredehorst
(2007).
Cloning and characterization of dihydrofolate reductase from a facultative alkaliphilic and halotolerant bacillus strain.
|
| |
Extremophiles,
11,
75-83.
|
 |
|
|
|
|
 |
T.Shirai,
K.Igarashi,
T.Ozawa,
H.Hagihara,
T.Kobayashi,
K.Ozaki,
and
S.Ito
(2007).
Ancestral sequence evolutionary trace and crystal structure analyses of alkaline alpha-amylase from Bacillus sp. KSM-1378 to clarify the alkaline adaptation process of proteins.
|
| |
Proteins,
66,
600-610.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.G.Kapetaniou,
A.Thanassoulas,
A.P.Dubnovitsky,
G.Nounesis,
and
A.C.Papageorgiou
(2006).
Effect of pH on the structure and stability of Bacillus circulans ssp. alkalophilus phosphoserine aminotransferase: thermodynamic and crystallographic studies.
|
| |
Proteins,
63,
742-753.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Manikandan,
A.Bhardwaj,
N.Gupta,
N.K.Lokanath,
A.Ghosh,
V.S.Reddy,
and
S.Ramakumar
(2006).
Crystal structures of native and xylosaccharide-bound alkali thermostable xylanase from an alkalophilic Bacillus sp. NG-27: structural insights into alkalophilicity and implications for adaptation to polyextreme conditions.
|
| |
Protein Sci,
15,
1951-1960.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.P.Dubnovitsky,
R.B.Ravelli,
A.N.Popov,
and
A.C.Papageorgiou
(2005).
Strain relief at the active site of phosphoserine aminotransferase induced by radiation damage.
|
| |
Protein Sci,
14,
1498-1507.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Padan,
E.Bibi,
M.Ito,
and
T.A.Krulwich
(2005).
Alkaline pH homeostasis in bacteria: new insights.
|
| |
Biochim Biophys Acta,
1717,
67-88.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

