 |
PDBsum entry 1vpt

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Methyltransferase
|
PDB id
|
|

|

|
1vpt
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.1.1.57
- methyltransferase cap1.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA + S-adenosyl-L-methionine = a 5'-end (N(7)-methyl 5'-triphosphoguanosine)- (2'-O-methyl-ribonucleoside) in mRNA + S-adenosyl-L-homocysteine + H+
|
 |
 |
 |
 |
 |
5'-end (N(7)-methyl 5'-triphosphoguanosine)-ribonucleoside in mRNA
|
+
|
S-adenosyl-L-methionine
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
5'-end (N(7)-methyl 5'-triphosphoguanosine)- (2'-O-methyl-ribonucleoside) in mRNA
|
+
|
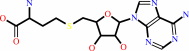 S-adenosyl-L-homocysteine
S-adenosyl-L-homocysteine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Cell
85:247-256
(1996)
|
|
PubMed id:
|
|
|
|

|
| |
|
The 1.85 A structure of vaccinia protein VP39: a bifunctional enzyme that participates in the modification of both mRNA ends.
|
|
A.E.Hodel,
P.D.Gershon,
X.Shi,
F.A.Quiocho.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
VP39 is a bifunctional vaccinia virus protein that acts as both an mRNA
cap-specific RNA 2'-O-methyltransferase and a poly(A) polymerase processivity
factor. Here, we report the 1.85 A crystal structure of a VP39 variant complexed
with its AdoMet cofactor. VP39 comprises a single core domain with structural
similarity to the catalytic domains of other methyltransferases. Surface
features and mutagenesis data suggest two possible RNA-binding sites with novel
underlying architecture, one of which forms a cleft spanning the region adjacent
to the methyltransferase active site. This report provides a prototypic
structure for an RNA methyltransferase, a protein that interacts with the mRNA
5' cap, and an intact poxvirus protein.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 4.
Figure 4. Stereo Diagram Showing Electron Density at the
AdoMet-Binding Site and the Interactions between AdoMet and
Nearby Residues of AS11The F[o]-F[c] electron density map,
contoured at 2.5 σ, was calculated at 1.85 Å from a model
that did not contain the AdoMet molecule. As shown, the AdoMet
molecule is clearly defined including its donor methyl group.
Residues appropriately positioned to make either hydrogen
bonding or van der Waals contact with AdoMet are shown. Carbon
atoms/bonds are colored gray/white; sulfur, yellow; nitrogen,
blue; and oxygen, red. The 13 potential hydrogen bonds whose
lengths were in the range 2.5–3.3 Å are represented by
dashed green lines. For clarity, G68 (whose backbone carbonyl
oxygen forms the near vertical hydrogen bond with the
α-ammonium group of the AdoMet methionine moiety) is not
labeled. One of the three potential hydrogen bonds to the 3′
OH of the AdoMet ribose is almost completely hidden in the view
shown.
|
 |
Figure 5.
Figure 5. Schematic Showing a Model for the Binding of a
5′-Capped RNA Strand to the Cleft and Methyltransferase Active
SiteThe cleft is viewed from an angle similar to that in Figure
3A, Figure 3D, and 3G. Important regions of the surface are
labeled, including the putative binding site for the terminal
(m^7G) nucleotide of cap 0 (cap binding); the position at which
the donor methyl of AdoMet protrudes into the cleft (donor
methyl); the active site and associated basic region (basic
region/active site); the missing 142–147 loop (which contains
basic side chains in wild-type VP39) and the hydrophobic pocket
at the distal end of the cleft. RNA nucleotides are represented
by dark ovals. An internucleotide spacing of  vert,
similar 5 Å per base was used, based upon the spacing
determined for single-stranded DNA bound to gp32 (in which DNA
bases were unstacked; [44]). vert,
similar 5 Å per base was used, based upon the spacing
determined for single-stranded DNA bound to gp32 (in which DNA
bases were unstacked; [44]).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Cell Press:
Cell
(1996,
85,
247-256)
copyright 1996.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.Decroly,
F.Ferron,
J.Lescar,
and
B.Canard
(2012).
Conventional and unconventional mechanisms for capping viral mRNA.
|
| |
Nat Rev Microbiol,
10,
51-65.
|
 |
|
|
|
|
 |
B.Selisko,
F.F.Peyrane,
B.Canard,
K.Alvarez,
and
E.Decroly
(2010).
Biochemical characterization of the (nucleoside-2'O)-methyltransferase activity of dengue virus protein NS5 using purified capped RNA oligonucleotides (7Me)GpppAC(n) and GpppAC(n).
|
| |
J Gen Virol,
91,
112-121.
|
 |
|
|
|
|
 |
A.M.Jansson,
E.Jakobsson,
P.Johansson,
V.Lantez,
B.Coutard,
X.de Lamballerie,
T.Unge,
and
T.A.Jones
(2009).
Structure of the methyltransferase domain from the Modoc virus, a flavivirus with no known vector.
|
| |
Acta Crystallogr D Biol Crystallogr,
65,
796-803.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Van Vliet,
M.R.Mohamed,
L.Zhang,
N.Y.Villa,
S.J.Werden,
J.Liu,
and
G.McFadden
(2009).
Poxvirus proteomics and virus-host protein interactions.
|
| |
Microbiol Mol Biol Rev,
73,
730-749.
|
 |
|
|
|
|
 |
B.Mittra,
J.R.Zamudio,
J.M.Bujnicki,
J.Stepinski,
E.Darzynkiewicz,
D.A.Campbell,
and
N.R.Sturm
(2008).
The TbMTr1 Spliced Leader RNA Cap 1 2 '-O-Ribose Methyltransferase from Trypanosoma brucei Acts with Substrate Specificity.
|
| |
J Biol Chem,
283,
3161-3172.
|
 |
|
|
|
|
 |
E.Decroly,
I.Imbert,
B.Coutard,
M.Bouvet,
B.Selisko,
K.Alvarez,
A.E.Gorbalenya,
E.J.Snijder,
and
B.Canard
(2008).
Coronavirus nonstructural protein 16 is a cap-0 binding enzyme possessing (nucleoside-2'O)-methyltransferase activity.
|
| |
J Virol,
82,
8071-8084.
|
 |
|
|
|
|
 |
H.Dong,
S.Ren,
B.Zhang,
Y.Zhou,
F.Puig-Basagoiti,
H.Li,
and
P.Y.Shi
(2008).
West Nile virus methyltransferase catalyzes two methylations of the viral RNA cap through a substrate-repositioning mechanism.
|
| |
J Virol,
82,
4295-4307.
|
 |
|
|
|
|
 |
M.N.Becker,
T.M.Todd,
and
R.W.Moyer
(2008).
An Amsacta moorei entomopoxvirus ortholog of the poly(A) polymerase small subunit exhibits methyltransferase activity and is non-essential for virus growth.
|
| |
Virology,
375,
624-636.
|
 |
|
|
|
|
 |
S.E.Galloway,
P.E.Richardson,
and
G.W.Wertz
(2008).
Analysis of a structural homology model of the 2'-O-ribose methyltransferase domain within the vesicular stomatitis virus L protein.
|
| |
Virology,
382,
69-82.
|
 |
|
|
|
|
 |
Y.Li,
and
L.A.Guarino
(2008).
Roles of LEF-4 and PTP/BVP RNA triphosphatases in processing of baculovirus late mRNAs.
|
| |
J Virol,
82,
5573-5583.
|
 |
|
|
|
|
 |
E.Mastrangelo,
M.Bollati,
M.Milani,
B.Selisko,
F.Peyrane,
B.Canard,
G.Grard,
X.de Lamballerie,
and
M.Bolognesi
(2007).
Structural bases for substrate recognition and activity in Meaban virus nucleoside-2'-O-methyltransferase.
|
| |
Protein Sci,
16,
1133-1145.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Y.Zhou,
D.Ray,
Y.Zhao,
H.Dong,
S.Ren,
Z.Li,
Y.Guo,
K.A.Bernard,
P.Y.Shi,
and
H.Li
(2007).
Structure and function of flavivirus NS5 methyltransferase.
|
| |
J Virol,
81,
3891-3903.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.Ray,
A.Shah,
M.Tilgner,
Y.Guo,
Y.Zhao,
H.Dong,
T.S.Deas,
Y.Zhou,
H.Li,
and
P.Y.Shi
(2006).
West Nile virus 5'-cap structure is formed by sequential guanine N-7 and ribose 2'-O methylations by nonstructural protein 5.
|
| |
J Virol,
80,
8362-8370.
|
 |
|
|
|
|
 |
G.K.Arhin,
H.Li,
E.Ullu,
and
C.Tschudi
(2006).
A protein related to the vaccinia virus cap-specific methyltransferase VP39 is involved in cap 4 modification in Trypanosoma brucei.
|
| |
RNA,
12,
53-62.
|
 |
|
|
|
|
 |
J.Li,
J.T.Wang,
and
S.P.Whelan
(2006).
A unique strategy for mRNA cap methylation used by vesicular stomatitis virus.
|
| |
Proc Natl Acad Sci U S A,
103,
8493-8498.
|
 |
|
|
|
|
 |
J.R.Zamudio,
B.Mittra,
G.M.Zeiner,
M.Feder,
J.M.Bujnicki,
N.R.Sturm,
and
D.A.Campbell
(2006).
Complete cap 4 formation is not required for viability in Trypanosoma brucei.
|
| |
Eukaryot Cell,
5,
905-915.
|
 |
|
|
|
|
 |
M.P.Hall,
and
C.K.Ho
(2006).
Functional characterization of a 48 kDa Trypanosoma brucei cap 2 RNA methyltransferase.
|
| |
Nucleic Acids Res,
34,
5594-5602.
|
 |
|
|
|
|
 |
A.Alexandrov,
E.J.Grayhack,
and
E.M.Phizicky
(2005).
tRNA m7G methyltransferase Trm8p/Trm82p: evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p.
|
| |
RNA,
11,
821-830.
|
 |
|
|
|
|
 |
J.Li,
E.C.Fontaine-Rodriguez,
and
S.P.Whelan
(2005).
Amino acid residues within conserved domain VI of the vesicular stomatitis virus large polymerase protein essential for mRNA cap methyltransferase activity.
|
| |
J Virol,
79,
13373-13384.
|
 |
|
|
|
|
 |
V.Z.Grdzelishvili,
S.Smallwood,
D.Tower,
R.L.Hall,
D.M.Hunt,
and
S.A.Moyer
(2005).
A single amino acid change in the L-polymerase protein of vesicular stomatitis virus completely abolishes viral mRNA cap methylation.
|
| |
J Virol,
79,
7327-7337.
|
 |
|
|
|
|
 |
J.Hager,
B.L.Staker,
and
U.Jakob
(2004).
Substrate binding analysis of the 23S rRNA methyltransferase RrmJ.
|
| |
J Bacteriol,
186,
6634-6642.
|
 |
|
|
|
|
 |
M.M.Slutsky,
and
E.N.Marsh
(2004).
Cation-pi interactions studied in a model coiled-coil peptide.
|
| |
Protein Sci,
13,
2244-2251.
|
 |
|
|
|
|
 |
X.Wu,
and
L.A.Guarino
(2003).
Autographa californica nucleopolyhedrovirus orf69 encodes an RNA cap (nucleoside-2'-O)-methyltransferase.
|
| |
J Virol,
77,
3430-3440.
|
 |
|
|
|
|
 |
A.Oguro,
L.Johnson,
and
P.D.Gershon
(2002).
Path of an RNA ligand around the surface of the vaccinia VP39 subunit of its cognate VP39-VP55 protein heterodimer.
|
| |
Chem Biol,
9,
679-690.
|
 |
|
|
|
|
 |
C.M.Groft,
and
S.K.Burley
(2002).
Recognition of eIF4G by rotavirus NSP3 reveals a basis for mRNA circularization.
|
| |
Mol Cell,
9,
1273-1283.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
F.Ferron,
S.Longhi,
B.Henrissat,
and
B.Canard
(2002).
Viral RNA-polymerases -- a predicted 2'-O-ribose methyltransferase domain shared by all Mononegavirales.
|
| |
Trends Biochem Sci,
27,
222-224.
|
 |
|
|
|
|
 |
G.D.Markham,
P.O.Norrby,
and
C.W.Bock
(2002).
S-adenosylmethionine conformations in solution and in protein complexes: conformational influences of the sulfonium group.
|
| |
Biochemistry,
41,
7636-7646.
|
 |
|
|
|
|
 |
H.Hori,
T.Suzuki,
K.Sugawara,
Y.Inoue,
T.Shibata,
S.Kuramitsu,
S.Yokoyama,
T.Oshima,
and
K.Watanabe
(2002).
Identification and characterization of tRNA (Gm18) methyltransferase from Thermus thermophilus HB8: domain structure and conserved amino acid sequence motifs.
|
| |
Genes Cells,
7,
259-272.
|
 |
|
|
|
|
 |
J.Hager,
B.L.Staker,
H.Bugl,
and
U.Jakob
(2002).
Active site in RrmJ, a heat shock-induced methyltransferase.
|
| |
J Biol Chem,
277,
41978-41986.
|
 |
|
|
|
|
 |
M.P.Egloff,
D.Benarroch,
B.Selisko,
J.L.Romette,
and
B.Canard
(2002).
An RNA cap (nucleoside-2'-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization.
|
| |
EMBO J,
21,
2757-2768.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
O.Nureki,
M.Shirouzu,
K.Hashimoto,
R.Ishitani,
T.Terada,
M.Tamakoshi,
T.Oshima,
M.Chijimatsu,
K.Takio,
D.G.Vassylyev,
T.Shibata,
Y.Inoue,
S.Kuramitsu,
and
S.Yokoyama
(2002).
An enzyme with a deep trefoil knot for the active-site architecture.
|
| |
Acta Crystallogr D Biol Crystallogr,
58,
1129-1137.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
X.Cheng,
and
R.J.Roberts
(2001).
AdoMet-dependent methylation, DNA methyltransferases and base flipping.
|
| |
Nucleic Acids Res,
29,
3784-3795.
|
 |
|
|
|
|
 |
F.A.Quiocho,
G.Hu,
and
P.D.Gershon
(2000).
Structural basis of mRNA cap recognition by proteins.
|
| |
Curr Opin Struct Biol,
10,
78-86.
|
 |
|
|
|
|
 |
H.Bügl,
E.B.Fauman,
B.L.Staker,
F.Zheng,
S.R.Kushner,
M.A.Saper,
J.C.Bardwell,
and
U.Jakob
(2000).
RNA methylation under heat shock control.
|
| |
Mol Cell,
6,
349-360.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Wang,
D.Boisvert,
K.K.Kim,
R.Kim,
and
S.H.Kim
(2000).
Crystal structure of a fibrillarin homologue from Methanococcus jannaschii, a hyperthermophile, at 1.6 A resolution.
|
| |
EMBO J,
19,
317-323.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Niewmierzycka,
and
S.Clarke
(1999).
S-Adenosylmethionine-dependent methylation in Saccharomyces cerevisiae. Identification of a novel protein arginine methyltransferase.
|
| |
J Biol Chem,
274,
814-824.
|
 |
|
|
|
|
 |
C.Schalk-Hihi,
and
G.D.Markham
(1999).
The conformations of a substrate and a product bound to the active site of S-adenosylmethionine synthetase.
|
| |
Biochemistry,
38,
2542-2550.
|
 |
|
|
|
|
 |
G.Hu,
P.D.Gershon,
A.E.Hodel,
and
F.A.Quiocho
(1999).
mRNA cap recognition: dominant role of enhanced stacking interactions between methylated bases and protein aromatic side chains.
|
| |
Proc Natl Acad Sci U S A,
96,
7149-7154.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Cavaillé,
F.Chetouani,
and
J.P.Bachellerie
(1999).
The yeast Saccharomyces cerevisiae YDL112w ORF encodes the putative 2'-O-ribose methyltransferase catalyzing the formation of Gm18 in tRNAs.
|
| |
RNA,
5,
66-81.
|
 |
|
|
|
|
 |
Y.Hu,
J.Komoto,
Y.Huang,
T.Gomi,
H.Ogawa,
Y.Takata,
M.Fujioka,
and
F.Takusagawa
(1999).
Crystal structure of S-adenosylhomocysteine hydrolase from rat liver.
|
| |
Biochemistry,
38,
8323-8333.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.M.Reeve,
S.D.Breazeale,
and
C.A.Townsend
(1998).
Purification, characterization, and cloning of an S-adenosylmethionine-dependent 3-amino-3-carboxypropyltransferase in nocardicin biosynthesis.
|
| |
J Biol Chem,
273,
30695-30703.
|
 |
|
|
|
|
 |
C.Schmutte,
and
P.A.Jones
(1998).
Involvement of DNA methylation in human carcinogenesis.
|
| |
Biol Chem,
379,
377-388.
|
 |
|
|
|
|
 |
D.E.Bussiere,
S.W.Muchmore,
C.G.Dealwis,
G.Schluckebier,
V.L.Nienaber,
R.P.Edalji,
K.A.Walter,
U.S.Ladror,
T.F.Holzman,
and
C.Abad-Zapatero
(1998).
Crystal structure of ErmC', an rRNA methyltransferase which mediates antibiotic resistance in bacteria.
|
| |
Biochemistry,
37,
7103-7112.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.K.O'Reilly,
Z.Wang,
R.French,
and
C.C.Kao
(1998).
Interactions between the structural domains of the RNA replication proteins of plant-infecting RNA viruses.
|
| |
J Virol,
72,
7160-7169.
|
 |
|
|
|
|
 |
E.Rom,
H.C.Kim,
A.C.Gingras,
J.Marcotrigiano,
D.Favre,
H.Olsen,
S.K.Burley,
and
N.Sonenberg
(1998).
Cloning and characterization of 4EHP, a novel mammalian eIF4E-related cap-binding protein.
|
| |
J Biol Chem,
273,
13104-13109.
|
 |
|
|
|
|
 |
H.L.Schubert,
K.S.Wilson,
E.Raux,
S.C.Woodcock,
and
M.J.Warren
(1998).
The X-ray structure of a cobalamin biosynthetic enzyme, cobalt-precorrin-4 methyltransferase.
|
| |
Nat Struct Biol,
5,
585-592.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Ogawa,
T.Gomi,
F.Takusagawa,
and
M.Fujioka
(1998).
Structure, function and physiological role of glycine N-methyltransferase.
|
| |
Int J Biochem Cell Biol,
30,
13-26.
|
 |
|
|
|
|
 |
M.I.Vázquez,
G.Rivas,
D.Cregut,
L.Serrano,
and
M.Esteban
(1998).
The vaccinia virus 14-kilodalton (A27L) fusion protein forms a triple coiled-coil structure and interacts with the 21-kilodalton (A17L) virus membrane protein through a C-terminal alpha-helix.
|
| |
J Virol,
72,
10126-10137.
|
 |
|
|
|
|
 |
M.Roth,
S.Helm-Kruse,
T.Friedrich,
and
A.Jeltsch
(1998).
Functional roles of conserved amino acid residues in DNA methyltransferases investigated by site-directed mutagenesis of the EcoRV adenine-N6-methyltransferase.
|
| |
J Biol Chem,
273,
17333-17342.
|
 |
|
|
|
|
 |
N.Sonenberg,
S.K.Burley,
and
A.C.Gingras
(1998).
RNA chiropractics.
|
| |
Nat Struct Biol,
5,
172-174.
|
 |
|
|
|
|
 |
P.H.Tran,
Z.R.Korszun,
S.Cerritelli,
S.S.Springhorn,
and
S.A.Lacks
(1998).
Crystal structure of the DpnM DNA adenine methyltransferase from the DpnII restriction system of streptococcus pneumoniae bound to S-adenosylmethionine.
|
| |
Structure,
6,
1563-1575.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.W.Lockless,
H.T.Cheng,
A.E.Hodel,
F.A.Quiocho,
and
P.D.Gershon
(1998).
Recognition of capped RNA substrates by VP39, the vaccinia virus-encoded mRNA cap-specific 2'-O-methyltransferase.
|
| |
Biochemistry,
37,
8564-8574.
|
 |
|
|
|
|
 |
A.E.Hodel,
P.D.Gershon,
X.Shi,
S.M.Wang,
and
F.A.Quiocho
(1997).
Specific protein recognition of an mRNA cap through its alkylated base.
|
| |
Nat Struct Biol,
4,
350-354.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.V.Efimov
(1997).
Structural trees for protein superfamilies.
|
| |
Proteins,
28,
241-260.
|
 |
|
|
|
|
 |
G.Varani
(1997).
A cap for all occasions.
|
| |
Structure,
5,
855-858.
|
 |
|
|
|
|
 |
L.Yu,
A.M.Petros,
A.Schnuchel,
P.Zhong,
J.M.Severin,
K.Walter,
T.F.Holzman,
and
S.W.Fesik
(1997).
Solution structure of an rRNA methyltransferase (ErmAM) that confers macrolide-lincosamide-streptogramin antibiotic resistance.
|
| |
Nat Struct Biol,
4,
483-489.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Djordjevic,
and
A.M.Stock
(1997).
Crystal structure of the chemotaxis receptor methyltransferase CheR suggests a conserved structural motif for binding S-adenosylmethionine.
|
| |
Structure,
5,
545-558.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Ahola,
P.Laakkonen,
H.Vihinen,
and
L.Kääriäinen
(1997).
Critical residues of Semliki Forest virus RNA capping enzyme involved in methyltransferase and guanylyltransferase-like activities.
|
| |
J Virol,
71,
392-397.
|
 |
|
|
|
|
 |
X.Shi,
T.G.Bernhardt,
S.M.Wang,
and
P.D.Gershon
(1997).
The surface region of the bifunctional vaccinia RNA modifying protein VP39 that interfaces with Poly(A) polymerase is remote from the RNA binding cleft used for its mRNA 5' cap methylation function.
|
| |
J Biol Chem,
272,
23292-23302.
|
 |
|
|
|
|
 |
H.C.Nelson,
and
T.H.Bestor
(1996).
Base eversion and shuffling by DNA methyltransferases.
|
| |
Chem Biol,
3,
419-423.
|
 |
|
|
|
|
 |
M.M.Dixon,
S.Huang,
R.G.Matthews,
and
M.Ludwig
(1996).
The structure of the C-terminal domain of methionine synthase: presenting S-adenosylmethionine for reductive methylation of B12.
|
| |
Structure,
4,
1263-1275.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

