 |
PDBsum entry 1vjo

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.6.1.44
- alanine--glyoxylate transaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
glyoxylate + L-alanine = glycine + pyruvate
|
 |
 |
 |
 |
 |
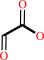 glyoxylate
glyoxylate
|
+
|
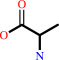 L-alanine
L-alanine
|
=
|
 glycine
glycine
|
+
|
 pyruvate
pyruvate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
corresponds exactly
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proteins
58:971-975
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of an alanine-glyoxylate aminotransferase from Anabaena sp. at 1.70 A resolution reveals a noncovalently linked PLP cofactor.
|
|
G.W.Han,
R.Schwarzenbacher,
R.Page,
L.Jaroszewski,
P.Abdubek,
E.Ambing,
T.Biorac,
J.M.Canaves,
H.J.Chiu,
X.Dai,
A.M.Deacon,
M.DiDonato,
M.A.Elsliger,
A.Godzik,
C.Grittini,
S.K.Grzechnik,
J.Hale,
E.Hampton,
J.Haugen,
M.Hornsby,
H.E.Klock,
E.Koesema,
A.Kreusch,
P.Kuhn,
S.A.Lesley,
I.Levin,
D.McMullan,
T.M.McPhillips,
M.D.Miller,
A.Morse,
K.Moy,
E.Nigoghossian,
J.Ouyang,
J.Paulsen,
K.Quijano,
R.Reyes,
E.Sims,
G.Spraggon,
R.C.Stevens,
H.van den Bedem,
J.Velasquez,
J.Vincent,
F.von Delft,
X.Wang,
B.West,
A.White,
G.Wolf,
Q.Xu,
O.Zagnitko,
K.O.Hodgson,
J.Wooley,
I.A.Wilson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. Crystal structure of AGT from Anabaena sp. (A) Stereo
ribbon diagram of Anabena sp. AGT color-coded from N-terminus
(blue) to C-terminus (red), showing the domain organization and
location of the putative active site (PLP molecule shown in
ball-and-stick representation). Helices H1-H15 and  -strands
( -strands
(  1- 1-
 12),
as well as 12),
as well as  -sheets
A, B, and C are indicated. (B) Diagram showing the secondary
structure elements in Anabaena sp. AGT superimposed on its
primary sequence. The -sheets
A, B, and C are indicated. (B) Diagram showing the secondary
structure elements in Anabaena sp. AGT superimposed on its
primary sequence. The  -sheet
designations are indicated by a red A, B, and C. Above each -sheet
designations are indicated by a red A, B, and C. Above each  -strand, -strand,
 -bulges
and -bulges
and  -turns
are indicated. -turns
are indicated.  -hairpins
are depicted as red loops. Disordered regions are depicted by a
dashed line, with the corresponding sequence in brackets. -hairpins
are depicted as red loops. Disordered regions are depicted by a
dashed line, with the corresponding sequence in brackets.
|
 |
Figure 2.
Figure 2. (A) Ribbon diagram of the Anabaena sp. AGT dimer. The
N-terminal segment (red), N-terminal domain (green), and
C-terminal domain (blue) are indicated for chain A. (B)
Superposition of Anabaena sp. AGT (blue) and human AGT (PDB
code: 1h0c; gray). The corresponding bound PLP and LLP
(lysine-pyridoxal-5  -phosphate)
ligands are shown in ball-and-stick representation, and regions
of structural difference are labeled. (C) The PLP molecule bound
to the active site of Anabaena sp. AGT and interacting residues
are shown in ball-and-stick representation. 2Fo-Fc density for
PLP contoured at 1 -phosphate)
ligands are shown in ball-and-stick representation, and regions
of structural difference are labeled. (C) The PLP molecule bound
to the active site of Anabaena sp. AGT and interacting residues
are shown in ball-and-stick representation. 2Fo-Fc density for
PLP contoured at 1  is
shown in blue. (D) Superposition of the active sites with the
PLP and LLP molecules and interacting residues from Anabaena sp.
AGT (blue) and their counterparts in human AGT (gray, residues
labeled in brackets) in ball-and-stick representation. is
shown in blue. (D) Superposition of the active sites with the
PLP and LLP molecules and interacting residues from Anabaena sp.
AGT (blue) and their counterparts in human AGT (gray, residues
labeled in brackets) in ball-and-stick representation.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from John Wiley & Sons, Inc.:
Proteins
(2005,
58,
971-975)
copyright 2005.
|
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.Panjikar,
V.Parthasarathy,
V.S.Lamzin,
M.S.Weiss,
and
P.A.Tucker
(2009).
On the combination of molecular replacement and single-wavelength anomalous diffraction phasing for automated structure determination.
|
| |
Acta Crystallogr D Biol Crystallogr,
65,
1089-1097.
|
 |
|
|
|
|
 |
H.Sakuraba,
K.Yoneda,
K.Takeuchi,
H.Tsuge,
N.Katunuma,
and
T.Ohshima
(2008).
Structure of an archaeal alanine:glyoxylate aminotransferase.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
696-699.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Y.Yoshikane,
N.Yokochi,
M.Yamasaki,
K.Mizutani,
K.Ohnishi,
B.Mikami,
H.Hayashi,
and
T.Yagi
(2008).
Crystal structure of pyridoxamine-pyruvate aminotransferase from Mesorhizobium loti MAFF303099.
|
| |
J Biol Chem,
283,
1120-1127.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Lima,
R.Khristoforov,
C.Momany,
and
R.S.Phillips
(2007).
Crystal structure of Homo sapiens kynureninase.
|
| |
Biochemistry,
46,
2735-2744.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Q.Han,
H.Robinson,
Y.G.Gao,
N.Vogelaar,
S.R.Wilson,
M.Rizzi,
and
J.Li
(2006).
Crystal structures of Aedes aegypti alanine glyoxylate aminotransferase.
|
| |
J Biol Chem,
281,
37175-37182.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

