 |
PDBsum entry 1v7y

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.2.1.20
- tryptophan synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Tryptophan Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate + L-serine = D-glyceraldehyde 3-phosphate + L-tryptophan + H2O
|
 |
 |
 |
 |
 |
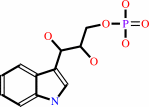 (1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate
(1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate
|
+
|
 L-serine
L-serine
|
=
|
 D-glyceraldehyde 3-phosphate
D-glyceraldehyde 3-phosphate
|
+
|
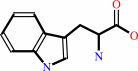 L-tryptophan
L-tryptophan
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
 Pyridoxal 5'-phosphate
Pyridoxal 5'-phosphate
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
44:1184-1192
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Conformational changes in the alpha-subunit coupled to binding of the beta 2-subunit of tryptophan synthase from Escherichia coli: crystal structure of the tryptophan synthase alpha-subunit alone.
|
|
K.Nishio,
Y.Morimoto,
M.Ishizuka,
K.Ogasahara,
T.Tsukihara,
K.Yutani.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
When the tryptophan synthase alpha- and beta(2)-subunits combine to form the
alpha(2)beta(2)-complex, the enzymatic activity of each subunit is stimulated by
1-2 orders of magnitude. To elucidate the structural basis of this mutual
activation, it is necessary to determine the structures of the alpha- and
beta-subunits alone and together with the alpha(2)beta(2)-complex. The crystal
structures of the tryptophan synthase alpha(2)beta(2)-complex from Salmonella
typhimurium (Stalpha(2)beta(2)-complex) have already been reported. However, the
structures of the subunit alone from mesophiles have not yet been determined.
The structure of the tryptophan synthase alpha-subunit alone from Escherichia
coli (Ecalpha-subunit) was determined by an X-ray crystallographic analysis at
2.3 A, which is the first report on the subunits alone from the mesophiles. The
biggest difference between the structures of the Ecalpha-subunit alone and the
alpha-subunit in the Stalpha(2)beta(2)-complex (Stalpha-subunit) was as follows.
Helix 2' in the Stalpha-subunit, including an active site residue (Asp60), was
changed to a flexible loop in the Ecalpha-subunit alone. The conversion of the
helix to a loop resulted in the collapse of the correct active site
conformation. This region is also an important part for the mutual activation in
the Stalpha(2)beta(2)-complex and interaction with the beta-subunit. These
results suggest that the formation of helix 2'that is essential for the
stimulation of the enzymatic activity of the alpha-subunit is constructed by the
induced-fit mode involved in conformational changes upon interaction between the
alpha- and beta-subunits. This also confirms the prediction of the
conformational changes based on the thermodynamic analysis for the association
between the alpha- and beta-subunits.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.Nishio,
K.Ogasahara,
Y.Morimoto,
T.Tsukihara,
S.J.Lee,
and
K.Yutani
(2010).
Large conformational changes in the Escherichia coli tryptophan synthase beta(2) subunit upon pyridoxal 5'-phosphate binding.
|
| |
FEBS J,
277,
2157-2170.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Fu,
G.R.Grimsley,
A.Razvi,
J.M.Scholtz,
and
C.N.Pace
(2009).
Increasing protein stability by improving beta-turns.
|
| |
Proteins,
77,
491-498.
|
 |
|
|
|
|
 |
X.Yang,
R.Vadrevu,
Y.Wu,
and
C.R.Matthews
(2007).
Long-range side-chain-main-chain interactions play crucial roles in stabilizing the (betaalpha)8 barrel motif of the alpha subunit of tryptophan synthase.
|
| |
Protein Sci,
16,
1398-1409.
|
 |
|
|
|
|
 |
Y.Wu,
R.Vadrevu,
S.Kathuria,
X.Yang,
and
C.R.Matthews
(2007).
A tightly packed hydrophobic cluster directs the formation of an off-pathway sub-millisecond folding intermediate in the alpha subunit of tryptophan synthase, a TIM barrel protein.
|
| |
J Mol Biol,
366,
1624-1638.
|
 |
|
|
|
|
 |
S.Raboni,
S.Bettati,
and
A.Mozzarelli
(2005).
Identification of the geometric requirements for allosteric communication between the alpha- and beta-subunits of tryptophan synthase.
|
| |
J Biol Chem,
280,
13450-13456.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

