 |
PDBsum entry 1v5x

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.5.3.1.24
- phosphoribosylanthranilate isomerase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Tryptophan Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N-(5-phospho-beta-D-ribosyl)anthranilate = 1-(2-carboxyphenylamino)-1- deoxy-D-ribulose 5-phosphate
|
 |
 |
 |
 |
 |
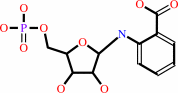 N-(5-phospho-beta-D-ribosyl)anthranilate
N-(5-phospho-beta-D-ribosyl)anthranilate
|
=
|
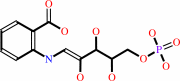 1-(2-carboxyphenylamino)-1- deoxy-D-ribulose 5-phosphate
1-(2-carboxyphenylamino)-1- deoxy-D-ribulose 5-phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Biochem (tokyo)
137:569-578
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Stabilization due to dimer formation of phosphoribosyl anthranilate isomerase from Thermus thermophilus HB8: X-ray Analysis and DSC experiments.
|
|
J.Taka,
K.Ogasahara,
J.Jeyakanthan,
N.Kunishima,
C.Kuroishi,
M.Sugahara,
S.Yokoyama,
K.Yutani.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of phosphoribosyl anthranilate isomerase (PRAI) from
Thermus thermophilus HB8 (TtPRAI) was solved at 2.0 A resolution. The overall
structure of TtPRAI with a dimeric structure was quite similar to that of PRAI
from Thermotoga maritima (TmPRAI). In order to elucidate the stabilization
mechanism of TtPRAI, its physicochemical properties were examined using DSC, CD,
and analytical centrifugation at various pHs in relation to the
association-dissociation of the subunits. Based on the experimental results for
TtPRAI and the structural information on TtPRAI and TmPRAI, we found that: (i)
the denaturation of TtPRAI at acidic pH is correlated with the dissociation of
its dimeric form; (ii) the hydrophobic interaction of TtPRAI in the monomer
structure is slightly greater than that of TmPRAI, but dimer interface of the
TmPRAI is remarkably greater; (iii) the contributions of hydrogen bonds and ion
bonds to the stability are similar to each other; and (iv) destabilization due
to the presence of cavities in TtPRAI is greater than that of TmPRAI in both the
monomer and dimer structures.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.Shen,
X.Xu,
H.Wu,
L.Peng,
Y.Zhang,
J.Song,
and
Q.Su
(2011).
Metal ion binding to anticoagulation factor II from the venom of Agkistrodon acutus: stabilization of the structure and regulation of the binding affinity to activated coagulation factor X.
|
| |
J Biol Inorg Chem,
16,
523-537.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

