 |
PDBsum entry 1v04

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Serum paraoxonase by directed evolution
|
|
Structure:
|
 |
Serum paraoxonase/arylesterase 1. Chain: a. Synonym: a-esterase 1, serum aryldialkylphosphatase 1, pon1, aromatic esterase 1. Engineered: yes. Other_details: paraoxonase-1 (pon1)
|
|
Source:
|
 |
Homo sapiens, oryctolagus cuniculus, mus musculus, rattus rattus. Organism_taxid: 9606, 9986, 10090, 10117. Expressed in: escherichia coli. Expression_system_taxid: 562. Other_details: from shuffled genes of human, rabbit, mouse and rat paraoxonase
|
|
Resolution:
|
 |
|
2.20Å
|
R-factor:
|
0.186
|
R-free:
|
0.217
|
|
|
Authors:
|
 |
M.Harel,A.Aharoni,L.Gaidukov,B.Brumshtein,O.Khersonsky,S.Yagur, R.Meged,H.Dvir,R.B.G.Ravelli,A.Mccarthy,L.Toker,I.Silman, J.L.Sussman,D.S.Tawfik
|
Key ref:

|
 |
M.Harel
et al.
(2004).
Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes.
Nat Struct Mol Biol,
11,
412-419.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
22-Mar-04
|
Release date:
|
23-Apr-04
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P27170
(PON1_RABIT) -
Serum paraoxonase/arylesterase 1 from Oryctolagus cuniculus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
359 a.a.
332 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 32 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.3.1.1.2
- arylesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a phenyl acetate + H2O = a phenol + acetate + H+
|
 |
 |
 |
 |
 |
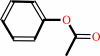 phenyl acetate
phenyl acetate
|
+
|
H2O
|
=
|
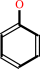 phenol
phenol
|
+
|
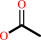 acetate
acetate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.3.1.1.81
- quorum-quenching N-acyl-homoserine lactonase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
an N-acyl-L-homoserine lactone + H2O = an N-acyl-L-homoserine + H+
|
 |
 |
 |
 |
 |
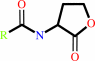 N-acyl-L-homoserine lactone
N-acyl-L-homoserine lactone
|
+
|
H2O
|
=
|
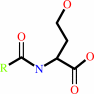 N-acyl-L-homoserine
N-acyl-L-homoserine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.3.1.8.1
- aryldialkylphosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
An aryl dialkyl phosphate + H2O = dialkyl phosphate + an aryl alcohol
|
 |
 |
 |
 |
 |
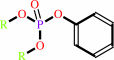 aryl dialkyl phosphate
aryl dialkyl phosphate
|
+
|
H2O
|
=
|
dialkyl phosphate
Bound ligand (Het Group name = )
matches with 71.43% similarity
|
+
|
aryl alcohol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Divalent cation
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nat Struct Mol Biol
11:412-419
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes.
|
|
M.Harel,
A.Aharoni,
L.Gaidukov,
B.Brumshtein,
O.Khersonsky,
R.Meged,
H.Dvir,
R.B.Ravelli,
A.McCarthy,
L.Toker,
I.Silman,
J.L.Sussman,
D.S.Tawfik.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Members of the serum paraoxonase (PON) family have been identified in mammals
and other vertebrates, and in invertebrates. PONs exhibit a wide range of
physiologically important hydrolytic activities, including drug metabolism and
detoxification of nerve agents. PON1 and PON3 reside on high-density lipoprotein
(HDL, 'good cholesterol') and are involved in the prevention of atherosclerosis.
We describe the first crystal structure of a PON family member, a variant of
PON1 obtained by directed evolution, at a resolution of 2.2 A. PON1 is a
six-bladed beta-propeller with a unique active site lid that is also involved in
HDL binding. The three-dimensional structure and directed evolution studies
permit a detailed description of PON1's active site and catalytic mechanism,
which are reminiscent of secreted phospholipase A2, and of the routes by which
PON family members diverged toward different substrate and reaction
selectivities.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2. PON1's active site viewed from above the propeller.
(a) Central tunnel of the propeller with the two calcium atoms,
and the side chains of the residues found to be mutated in the
newly evolved PON1 variants for esterase and lactonase (orange)
or for phosphotriesterase activity (yellow), including the R192Q
human polymorphism (in the rePON1-G2E6 variant, this position is
a lysine). The putative catalytic His-His dyad is red (see text
and Fig. 4). (b) A surface view of the active site. Lys70, Tyr71
and Phe347 are shown as sticks to permit a better view of the
active site. At the deepest point of the cavity lies the upper
calcium atom (Ca1, green) to which a phosphate ion is bound.
|
 |
Figure 6.
Figure 6. Proposed model for anchoring of PON1 to the surface of
HDL. (a) Tertiary structure cartoon of rePON1 showing its
exposed hydrophobic surfaces. N-terminal residues 7 -18, missing
in the crystal structure and predicted to be helical, were
modeled as part of H1. Denoted are all the hydrophobic residues
(leucine, phenylalanine, proline, isoleucine, tyrosine,
tryptophan and valine) appearing with accessible surface area
 20
┼2. (b) Hydrophobic residues proposed to be involved in HDL
anchoring (side chains yellow). The line defined by the side
chains of Tyr185, Phe 186, Tyr190, Trp194, Trp202 (helix H2 and
the adjacent loops) and Lys21 (helix H1) models the putative
interface between HDL's hydrophobic interior and the exterior
aqueous phase. The hydrophobic side chains of leucine and
phenylalanine residues of H1 are primarily within the apolar
region31. The active site and the selectivity-determining
residues (Table 2) are blue, and the proposed glycosylation
sites (Asn253 and Asn324) are red. 20
┼2. (b) Hydrophobic residues proposed to be involved in HDL
anchoring (side chains yellow). The line defined by the side
chains of Tyr185, Phe 186, Tyr190, Trp194, Trp202 (helix H2 and
the adjacent loops) and Lys21 (helix H1) models the putative
interface between HDL's hydrophobic interior and the exterior
aqueous phase. The hydrophobic side chains of leucine and
phenylalanine residues of H1 are primarily within the apolar
region31. The active site and the selectivity-determining
residues (Table 2) are blue, and the proposed glycosylation
sites (Asn253 and Asn324) are red.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nat Struct Mol Biol
(2004,
11,
412-419)
copyright 2004.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Devarajan,
N.Bourquard,
S.Hama,
M.Navab,
V.R.Grijalva,
S.Morvardi,
C.F.Clarke,
L.Vergnes,
K.Reue,
J.F.Teiber,
and
S.T.Reddy
(2011).
Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis.
|
| |
Antioxid Redox Signal,
14,
341-351.
|
 |
|
|
|
|
 |
H.J.Bouman,
E.Schömig,
J.W.van Werkum,
J.Velder,
C.M.Hackeng,
C.Hirschhäuser,
C.Waldmann,
H.G.Schmalz,
J.M.ten Berg,
and
D.Taubert
(2011).
Paraoxonase-1 is a major determinant of clopidogrel efficacy.
|
| |
Nat Med,
17,
110-116.
|
 |
|
|
|
|
 |
H.Tavori,
M.Aviram,
S.Khatib,
R.Musa,
D.Mannheim,
R.Karmeli,
and
J.Vaya
(2011).
Human carotid lesion linoleic acid hydroperoxide inhibits paraoxonase 1 (PON1) activity via reaction with PON1 free sulfhydryl cysteine 284.
|
| |
Free Radic Biol Med,
50,
148-156.
|
 |
|
|
|
|
 |
K.Huen,
L.Barcellos,
K.Beckman,
S.Rose,
B.Eskenazi,
and
N.Holland
(2011).
Effects of PON polymorphisms and haplotypes on molecular phenotype in Mexican-American mothers and children.
|
| |
Environ Mol Mutagen,
52,
105-116.
|
 |
|
|
|
|
 |
M.Valiyaveettil,
Y.Alamneh,
L.Biggemann,
I.Soojhawon,
H.A.Farag,
P.Agrawal,
B.P.Doctor,
and
M.P.Nambiar
(2011).
In vitro efficacy of paraoxonase 1 from multiple sources against various organophosphates.
|
| |
Toxicol In Vitro,
25,
905-913.
|
 |
|
|
|
|
 |
M.Valiyaveettil,
Y.Alamneh,
P.Rezk,
M.W.Perkins,
A.M.Sciuto,
B.P.Doctor,
and
M.P.Nambiar
(2011).
Recombinant paraoxonase 1 protects against sarin and soman toxicity following microinstillation inhalation exposure in guinea pigs.
|
| |
Toxicol Lett,
202,
203-208.
|
 |
|
|
|
|
 |
R.J.Boado,
E.K.Hui,
J.Z.Lu,
and
W.M.Pardridge
(2011).
CHO cell expression, long-term stability, and primate pharmacokinetics and brain uptake of an IgG-paroxonase-1 fusion protein.
|
| |
Biotechnol Bioeng,
108,
186-196.
|
 |
|
|
|
|
 |
S.P.Deakin,
S.Bioletto,
M.L.Bochaton-Piallat,
and
R.W.James
(2011).
HDL-associated paraoxonase-1 can redistribute to cell membranes and influence sensitivity to oxidative stress.
|
| |
Free Radic Biol Med,
50,
102-109.
|
 |
|
|
|
|
 |
S.Z.Fairchild,
M.W.Peterson,
A.Hamza,
C.G.Zhan,
D.M.Cerasoli,
and
W.E.Chang
(2011).
Computational characterization of how the VX nerve agent binds human serum paraoxonase 1.
|
| |
J Mol Model,
17,
97.
|
 |
|
|
|
|
 |
C.Zhang,
W.Peng,
M.Wang,
J.Zhu,
Y.Zang,
W.Shi,
J.Zhang,
and
J.Qin
(2010).
Studies on protective effects of human paraoxonases 1 and 3 on atherosclerosis in apolipoprotein E knockout mice.
|
| |
Gene Ther,
17,
626-633.
|
 |
|
|
|
|
 |
D.Ekinci,
M.Sentürk,
S.Beydemir,
O.I.Küfrevio─člu,
and
C.T.Supuran
(2010).
An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides.
|
| |
Chem Biol Drug Des,
76,
552-558.
|
 |
|
|
|
|
 |
J.G.Bogner-Strauss,
A.Prokesch,
F.Sanchez-Cabo,
D.Rieder,
H.Hackl,
K.Duszka,
A.Krogsdam,
B.Di Camillo,
E.Walenta,
A.Klatzer,
A.Lass,
M.Pinent,
W.C.Wong,
F.Eisenhaber,
and
Z.Trajanoski
(2010).
Reconstruction of gene association network reveals a transmembrane protein required for adipogenesis and targeted by PPAR╬│.
|
| |
Cell Mol Life Sci,
67,
4049-4064.
|
 |
|
|
|
|
 |
S.Ahmad,
J.J.Carter,
and
J.E.Scott
(2010).
A homogeneous cell-based assay for measurement of endogenous paraoxonase 1 activity.
|
| |
Anal Biochem,
400,
1-9.
|
 |
|
|
|
|
 |
S.M.Suzuki,
R.C.Stevens,
R.J.Richter,
T.B.Cole,
S.Park,
T.C.Otto,
D.M.Cerasoli,
D.E.Lenz,
and
C.E.Furlong
(2010).
Engineering Human PON1 in an E. coli Expression System.
|
| |
Adv Exp Med Biol,
660,
37-45.
|
 |
|
|
|
|
 |
D.A.Stoltz,
E.A.Ozer,
T.J.Recker,
M.Estin,
X.Yang,
D.M.Shih,
A.J.Lusis,
and
J.Zabner
(2009).
A common mutation in paraoxonase-2 results in impaired lactonase activity.
|
| |
J Biol Chem,
284,
35564-35571.
|
 |
|
|
|
|
 |
F.Lescai,
F.Marchegiani,
and
C.Franceschi
(2009).
PON1 is a longevity gene: results of a meta-analysis.
|
| |
Ageing Res Rev,
8,
277-284.
|
 |
|
|
|
|
 |
J.D.Bloom,
and
F.H.Arnold
(2009).
In the light of directed evolution: pathways of adaptive protein evolution.
|
| |
Proc Natl Acad Sci U S A,
106,
9995.
|
 |
|
|
|
|
 |
J.J.Regieli,
J.W.Jukema,
P.A.Doevendans,
A.H.Zwinderman,
J.J.Kastelein,
D.E.Grobbee,
and
Y.van der Graaf
(2009).
Paraoxonase variants relate to 10-year risk in coronary artery disease: impact of a high-density lipoprotein-bound antioxidant in secondary prevention.
|
| |
J Am Coll Cardiol,
54,
1238-1245.
|
 |
|
|
|
|
 |
K.A.Davis,
J.A.Crow,
H.W.Chambers,
E.C.Meek,
and
J.E.Chambers
(2009).
Racial differences in paraoxonase-1 (PON1): a factor in the health of southerners?
|
| |
Environ Health Perspect,
117,
1226-1231.
|
 |
|
|
|
|
 |
L.Gaidukov,
D.Bar,
S.Yacobson,
E.Naftali,
O.Kaufman,
R.Tabakman,
D.S.Tawfik,
and
E.Levy-Nissenbaum
(2009).
In vivo administration of BL-3050: highly stable engineered PON1-HDL complexes.
|
| |
BMC Clin Pharmacol,
9,
18.
|
 |
|
|
|
|
 |
M.M.Blum,
M.Mustyakimov,
H.Rüterjans,
K.Kehe,
B.P.Schoenborn,
P.Langan,
and
J.C.Chen
(2009).
Rapid determination of hydrogen positions and protonation states of diisopropyl fluorophosphatase by joint neutron and X-ray diffraction refinement.
|
| |
Proc Natl Acad Sci U S A,
106,
713-718.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
N.Martinelli,
D.Girelli,
O.Olivieri,
P.Guarini,
A.Bassi,
E.Trabetti,
S.Friso,
F.Pizzolo,
C.Bozzini,
I.Tenuti,
L.Annarumma,
R.Schiavon,
P.Franco Pignatti,
and
R.Corrocher
(2009).
Novel serum paraoxonase activity assays are associated with coronary artery disease.
|
| |
Clin Chem Lab Med,
47,
432-440.
|
 |
|
|
|
|
 |
P.Del Vecchio,
M.Elias,
L.Merone,
G.Graziano,
J.Dupuy,
L.Mandrich,
P.Carullo,
B.Fournier,
D.Rochu,
M.Rossi,
P.Masson,
E.Chabriere,
and
G.Manco
(2009).
Structural determinants of the high thermal stability of SsoPox from the hyperthermophilic archaeon Sulfolobus solfataricus.
|
| |
Extremophiles,
13,
461-470.
|
 |
|
|
|
|
 |
R.Romani,
G.E.De Medio,
S.di Tullio,
R.Lapalombella,
I.Pirisinu,
V.Margonato,
A.Veicsteinas,
M.Marini,
and
G.Rosi
(2009).
Modulation of paraoxonase 1 and 3 expression after moderate exercise training in the rat.
|
| |
J Lipid Res,
50,
2036-2045.
|
 |
|
|
|
|
 |
T.C.Otto,
C.K.Harsch,
D.T.Yeung,
T.J.Magliery,
D.M.Cerasoli,
and
D.E.Lenz
(2009).
Dramatic differences in organophosphorus hydrolase activity between human and chimeric recombinant mammalian paraoxonase-1 enzymes.
|
| |
Biochemistry,
48,
10416-10422.
|
 |
|
|
|
|
 |
X.Hu,
X.Jiang,
D.E.Lenz,
D.M.Cerasoli,
and
A.Wallqvist
(2009).
In silico analyses of substrate interactions with human serum paraoxonase 1.
|
| |
Proteins,
75,
486-498.
|
 |
|
|
|
|
 |
Z.Hashim,
A.Ilyas,
A.Saleem,
A.Salim,
and
S.Zarina
(2009).
Expression and activity of paraoxonase 1 in human cataractous lens tissue.
|
| |
Free Radic Biol Med,
46,
1089-1095.
|
 |
|
|
|
|
 |
A.L.Brown,
Z.Liao,
and
M.B.Goodman
(2008).
MEC-2 and MEC-6 in the Caenorhabditis elegans sensory mechanotransduction complex: auxiliary subunits that enable channel activity.
|
| |
J Gen Physiol,
131,
605-616.
|
 |
|
|
|
|
 |
D.K.Nomura,
K.Fujioka,
R.S.Issa,
A.M.Ward,
B.F.Cravatt,
and
J.E.Casida
(2008).
Dual roles of brain serine hydrolase KIAA1363 in ether lipid metabolism and organophosphate detoxification.
|
| |
Toxicol Appl Pharmacol,
228,
42-48.
|
 |
|
|
|
|
 |
F.Marchegiani,
M.Marra,
F.Olivieri,
M.Cardelli,
R.W.James,
M.Boemi,
and
C.Franceschi
(2008).
Paraoxonase 1: genetics and activities during aging.
|
| |
Rejuvenation Res,
11,
113-127.
|
 |
|
|
|
|
 |
M.Unür,
E.Demirez,
B.A─čaçhan,
U.Görmü┼č,
A.Ergen,
B.Dalan,
and
T.Isbir
(2008).
The relationship of oral disturbances of diabetes mellitus patients with paraoxonase gene polymorphisms.
|
| |
Cell Biochem Funct,
26,
870-873.
|
 |
|
|
|
|
 |
R.J.Boado,
Y.Zhang,
Y.Zhang,
Y.Wang,
and
W.M.Pardridge
(2008).
IgG-paraoxonase-1 fusion protein for targeted drug delivery across the human blood-brain barrier.
|
| |
Mol Pharm,
5,
1037-1043.
|
 |
|
|
|
|
 |
S.Yair,
B.Ofer,
E.Arik,
S.Shai,
R.Yossi,
D.Tzvika,
and
K.Amir
(2008).
Organophosphate degrading microorganisms and enzymes as biocatalysts in environmental and personal decontamination applications.
|
| |
Crit Rev Biotechnol,
28,
265-275.
|
 |
|
|
|
|
 |
T.L.Graves,
and
J.E.Scott
(2008).
A high throughput serum paraoxonase assay for discovery of small molecule modulators of PON1 activity.
|
| |
Curr Chem Genomics,
2,
51-61.
|
 |
|
|
|
|
 |
D.T.Yeung,
J.R.Smith,
R.E.Sweeney,
D.E.Lenz,
and
D.M.Cerasoli
(2007).
Direct detection of stereospecific soman hydrolysis by wild-type human serum paraoxonase.
|
| |
FEBS J,
274,
1183-1191.
|
 |
|
|
|
|
 |
F.Liao,
X.Y.Zhu,
Y.M.Wang,
Y.S.Zhao,
L.P.Zhu,
and
Y.P.Zuo
(2007).
Correlation of serum arylesterase activity on phenylacetate estimated by the integrated method to common classical biochemical indexes of liver damage.
|
| |
J Zhejiang Univ Sci B,
8,
237-241.
|
 |
|
|
|
|
 |
G.Amitai,
R.D.Gupta,
and
D.S.Tawfik
(2007).
Latent evolutionary potentials under the neutral mutational drift of an enzyme.
|
| |
HFSP J,
1,
67-78.
|
 |
|
|
|
|
 |
J.Stöckigt,
and
S.Panjikar
(2007).
Structural biology in plant natural product biosynthesis--architecture of enzymes from monoterpenoid indole and tropane alkaloid biosynthesis.
|
| |
Nat Prod Rep,
24,
1382-1400.
|
 |
|
|
|
|
 |
M.Harel,
B.Brumshtein,
R.Meged,
H.Dvir,
R.B.Ravelli,
A.McCarthy,
L.Toker,
I.Silman,
and
J.L.Sussman
(2007).
3-D structure of serum paraoxonase 1 sheds light on its activity, stability, solubility and crystallizability.
|
| |
Arh Hig Rada Toksikol,
58,
347-353.
|
 |
|
|
|
|
 |
M.Kataoka,
K.Honda,
K.Sakamoto,
and
S.Shimizu
(2007).
Microbial enzymes involved in lactone compound metabolism and their biotechnological applications.
|
| |
Appl Microbiol Biotechnol,
75,
257-266.
|
 |
|
|
|
|
 |
M.M.Blum,
A.Koglin,
H.Rüterjans,
B.Schoenborn,
P.Langan,
and
J.C.Chen
(2007).
Preliminary time-of-flight neutron diffraction study on diisopropyl fluorophosphatase (DFPase) from Loligo vulgaris.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
63,
42-45.
|
 |
|
|
|
|
 |
S.Kiranoglu,
S.Sinan,
N.Gencer,
F.Köckar,
and
O.Arslan
(2007).
In vivo effects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse.
|
| |
Biol Pharm Bull,
30,
1048-1051.
|
 |
|
|
|
|
 |
T.Kriska,
G.K.Marathe,
J.C.Schmidt,
T.M.McIntyre,
and
A.W.Girotti
(2007).
Phospholipase action of platelet-activating factor acetylhydrolase, but not paraoxonase-1, on long fatty acyl chain phospholipid hydroperoxides.
|
| |
J Biol Chem,
282,
100-108.
|
 |
|
|
|
|
 |
Y.H.Dong,
L.Y.Wang,
and
L.H.Zhang
(2007).
Quorum-quenching microbial infections: mechanisms and implications.
|
| |
Philos Trans R Soc Lond B Biol Sci,
362,
1201-1211.
|
 |
|
|
|
|
 |
Y.Tanaka,
K.Morikawa,
Y.Ohki,
M.Yao,
K.Tsumoto,
N.Watanabe,
T.Ohta,
and
I.Tanaka
(2007).
Structural and mutational analyses of Drp35 from Staphylococcus aureus: a possible mechanism for its lactonase activity.
|
| |
J Biol Chem,
282,
5770-5780.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Y.Tanaka,
T.Sasaki,
I.Kumagai,
Y.Yasutake,
M.Yao,
I.Tanaka,
and
K.Tsumoto
(2007).
Molecular properties of two proteins homologous to PduO-type ATP:cob(I)alamin adenosyltransferase from Sulfolobus tokodaii.
|
| |
Proteins,
68,
446-457.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
B.M.Liederer,
and
R.T.Borchardt
(2006).
Enzymes involved in the bioconversion of ester-based prodrugs.
|
| |
J Pharm Sci,
95,
1177-1195.
|
 |
|
|
|
|
 |
C.H.Park,
S.D.Nguyen,
M.R.Kim,
T.S.Jeong,
and
D.E.Sok
(2006).
Differential effect of lysophospholipids on activities of human plasma paraoxonase1, either soluble or lipid-bound.
|
| |
Lipids,
41,
371-380.
|
 |
|
|
|
|
 |
G.Amitai,
L.Gaidukov,
R.Adani,
S.Yishay,
G.Yacov,
M.Kushnir,
S.Teitlboim,
M.Lindenbaum,
P.Bel,
O.Khersonsky,
D.S.Tawfik,
and
H.Meshulam
(2006).
Enhanced stereoselective hydrolysis of toxic organophosphates by directly evolved variants of mammalian serum paraoxonase.
|
| |
FEBS J,
273,
1906-1919.
|
 |
|
|
|
|
 |
J.Zhu,
Y.Ze,
C.Zhang,
Y.Zang,
H.Lu,
P.Chu,
M.Sun,
and
J.Qin
(2006).
High-level expression of recombinant human paraoxonase 1 Q in silkworm larvae (Bombyx mori).
|
| |
Appl Microbiol Biotechnol,
72,
103-108.
|
 |
|
|
|
|
 |
L.F.Huang,
B.Su,
S.C.Jao,
K.T.Liu,
and
W.S.Li
(2006).
Aminopeptidase p mediated detoxification of organophosphonate analogues of sarin: mechanistic and stereochemical study at the phosphorus atom of the substrate.
|
| |
Chembiochem,
7,
506-514.
|
 |
|
|
|
|
 |
M.Rosenblat,
L.Gaidukov,
O.Khersonsky,
J.Vaya,
R.Oren,
D.S.Tawfik,
and
M.Aviram
(2006).
The catalytic histidine dyad of high density lipoprotein-associated serum paraoxonase-1 (PON1) is essential for PON1-mediated inhibition of low density lipoprotein oxidation and stimulation of macrophage cholesterol efflux.
|
| |
J Biol Chem,
281,
7657-7665.
|
 |
|
|
|
|
 |
O.Khersonsky,
and
D.S.Tawfik
(2006).
The histidine 115-histidine 134 dyad mediates the lactonase activity of mammalian serum paraoxonases.
|
| |
J Biol Chem,
281,
7649-7656.
|
 |
|
|
|
|
 |
W.F.Li,
M.H.Pan,
M.C.Chung,
C.K.Ho,
and
H.Y.Chuang
(2006).
Lead exposure is associated with decreased serum paraoxonase 1 (PON1) activity and genotypes.
|
| |
Environ Health Perspect,
114,
1233-1236.
|
 |
|
|
|
|
 |
A.Aharoni,
G.Amitai,
K.Bernath,
S.Magdassi,
and
D.S.Tawfik
(2005).
High-throughput screening of enzyme libraries: thiolactonases evolved by fluorescence-activated sorting of single cells in emulsion compartments.
|
| |
Chem Biol,
12,
1281-1289.
|
 |
|
|
|
|
 |
A.Aharoni,
L.Gaidukov,
O.Khersonsky,
S.McQ Gould,
C.Roodveldt,
and
D.S.Tawfik
(2005).
The 'evolvability' of promiscuous protein functions.
|
| |
Nat Genet,
37,
73-76.
|
 |
|
|
|
|
 |
B.Bryk,
L.BenMoyal-Segal,
E.Podoly,
O.Livnah,
A.Eisenkraft,
S.Luria,
A.Cohen,
Y.Yehezkelli,
A.Hourvitz,
and
H.Soreq
(2005).
Inherited and acquired interactions between ACHE and PON1 polymorphisms modulate plasma acetylcholinesterase and paraoxonase activities.
|
| |
J Neurochem,
92,
1216-1227.
|
 |
|
|
|
|
 |
D.T.Yeung,
D.E.Lenz,
and
D.M.Cerasoli
(2005).
Analysis of active-site amino-acid residues of human serum paraoxonase using competitive substrates.
|
| |
FEBS J,
272,
2225-2230.
|
 |
|
|
|
|
 |
K.Morikawa,
T.Hidaka,
H.Murakami,
H.Hayashi,
and
T.Ohta
(2005).
Staphylococcal Drp35 is the functional counterpart of the eukaryotic PONs.
|
| |
FEMS Microbiol Lett,
249,
185-190.
|
 |
|
|
|
|
 |
L.Merone,
L.Mandrich,
M.Rossi,
and
G.Manco
(2005).
A thermostable phosphotriesterase from the archaeon Sulfolobus solfataricus: cloning, overexpression and properties.
|
| |
Extremophiles,
9,
297-305.
|
 |
|
|
|
|
 |
M.Aviram,
and
M.Rosenblat
(2005).
Paraoxonases and cardiovascular diseases: pharmacological and nutritional influences.
|
| |
Curr Opin Lipidol,
16,
393-399.
|
 |
|
|
|
|
 |
R.W.James,
B.Kalix,
S.Bioletto,
and
M.C.Brulhart-Meynet
(2005).
Paraoxonase-1 promoter polymorphism C--107T and serum apolipoprotein AI interact to modulate serum paraoxonase-1 status.
|
| |
Pharmacogenet Genomics,
15,
441-446.
|
 |
|
|
|
|
 |
S.Searles Nielsen,
B.A.Mueller,
A.J.De Roos,
H.M.Viernes,
F.M.Farin,
and
H.Checkoway
(2005).
Risk of brain tumors in children and susceptibility to organophosphorus insecticides: the potential role of paraoxonase (PON1).
|
| |
Environ Health Perspect,
113,
909-913.
|
 |
|
|
|
|
 |
M.Aviram,
and
M.Rosenblat
(2004).
Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development.
|
| |
Free Radic Biol Med,
37,
1304-1316.
|
 |
|
|
|
|
 |
R.W.James,
and
S.P.Deakin
(2004).
The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity.
|
| |
Free Radic Biol Med,
37,
1986-1994.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

