 |
PDBsum entry 1uux

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.10.1.1
- molybdopterin molybdotransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
adenylyl-molybdopterin + molybdate = Mo-molybdopterin + AMP + H+
|
 |
 |
 |
 |
 |
adenylyl-molybdopterin
|
+
|
molybdate
|
=
|
Mo-molybdopterin
|
+
|
AMP
Bound ligand (Het Group name = )
matches with 74.07% similarity
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+) or Mg(2+)
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.2.7.7.75
- molybdopterin adenylyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
molybdopterin + ATP + H+ = adenylyl-molybdopterin + diphosphate
|
 |
 |
 |
 |
 |
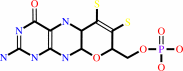 molybdopterin
molybdopterin
|
+
|
 ATP
ATP
|
+
|
H(+)
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
adenylyl-molybdopterin
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mn(2+) or Mg(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nature
430:803-806
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of the molybdopterin-bound Cnx1G domain links molybdenum and copper metabolism.
|
|
J.Kuper,
A.Llamas,
H.J.Hecht,
R.R.Mendel,
G.Schwarz.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The molybdenum cofactor is part of the active site of all molybdenum-dependent
enzymes, except nitrogenase. The molybdenum cofactor consists of molybdopterin,
a phosphorylated pyranopterin, with an ene-dithiolate coordinating molybdenum.
The same pyranopterin-based cofactor is involved in metal coordination of the
homologous tungsten-containing enzymes found in archea. The molybdenum cofactor
is synthesized by a highly conserved biosynthetic pathway. In plants, the
multidomain protein Cnx1 catalyses the insertion of molybdenum into
molybdopterin. The Cnx1 G domain (Cnx1G), whose crystal structure has been
determined in its apo form, binds molybdopterin with high affinity and
participates in the catalysis of molybdenum insertion. Here we present two
high-resolution crystal structures of Cnx1G in complex with molybdopterin and
with adenylated molybdopterin (molybdopterin-AMP), a mechanistically important
intermediate. Molybdopterin-AMP is the reaction product of Cnx1G and is
subsequently processed in a magnesium-dependent reaction by the amino-terminal E
domain of Cnx1 to yield active molybdenum cofactor. The unexpected
identification of copper bound to the molybdopterin dithiolate sulphurs in both
structures, coupled with the observed copper inhibition of Cnx1G activity,
provides a molecular link between molybdenum and copper metabolism.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1: Structure of the Cnx1G -MPT and Ser583Ala -MPT -AMP
complexes. a, b, Ribbon representation of Cnx1G (a) and
Ser583Ala (b).  -Helices
and -Helices
and  -strands
are shown in green and orange, respectively. Bound MPT and MPT
-AMP are shown in ball-and-stick notation and are covered by the
2F[o] -F[c] electron density contoured at 1.0 -strands
are shown in green and orange, respectively. Bound MPT and MPT
-AMP are shown in ball-and-stick notation and are covered by the
2F[o] -F[c] electron density contoured at 1.0  .
The difference density (F[o] -F[c]) is shown in red (3.5 .
The difference density (F[o] -F[c]) is shown in red (3.5  ).
c, d, Close-up views of the bound MPT (c) and AMP (d) regions in
the Ser583Ala structure. e, f, Molecular surface of Ser583Ala
with bound MPT -AMP showing, respectively, residues important
for MPT binding (red) and catalysis12,14 (blue), and the
electrostatic surface potential with ).
c, d, Close-up views of the bound MPT (c) and AMP (d) regions in
the Ser583Ala structure. e, f, Molecular surface of Ser583Ala
with bound MPT -AMP showing, respectively, residues important
for MPT binding (red) and catalysis12,14 (blue), and the
electrostatic surface potential with  11
kT as borders for electropositive (blue) and negative (red)
regions calculated with SYBIL and GRASP30. The catalytically
important residues Asp 515 and Asp 548 are highlighted. The
position of Mg2+ found in the homologous MoeA^22 domain is
superimposed. 11
kT as borders for electropositive (blue) and negative (red)
regions calculated with SYBIL and GRASP30. The catalytically
important residues Asp 515 and Asp 548 are highlighted. The
position of Mg2+ found in the homologous MoeA^22 domain is
superimposed.
|
 |
Figure 4.
Figure 4: Hypothetical mechanism of the molybdenum insertion
reaction. The mechanism of copper chelation is unknown. Cnx1G
adenylates Cu -MPT, a reaction that is thought to be cation
(M2+) dependent. Cnx1E subsequently cleaves the Cu -MPT -AMP
complex to activate and to transfer the molybdenum atom to MPT.
The function of copper is seen in the protection of the reactive
MPT dithiolate.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nature
(2004,
430,
803-806)
copyright 2004.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.Reiss,
and
R.Hahnewald
(2011).
Molybdenum cofactor deficiency: Mutations in GPHN, MOCS1, and MOCS2.
|
| |
Hum Mutat,
32,
10-18.
|
 |
|
|
|
|
 |
J.Teschner,
N.Lachmann,
J.Schulze,
M.Geisler,
K.Selbach,
J.Santamaria-Araujo,
J.Balk,
R.R.Mendel,
and
F.Bittner
(2010).
A novel role for Arabidopsis mitochondrial ABC transporter ATM3 in molybdenum cofactor biosynthesis.
|
| |
Plant Cell,
22,
468-480.
|
 |
|
|
|
|
 |
Y.Zhang,
and
V.N.Gladyshev
(2010).
General trends in trace element utilization revealed by comparative genomic analyses of Co, Cu, Mo, Ni, and Se.
|
| |
J Biol Chem,
285,
3393-3405.
|
 |
|
|
|
|
 |
G.Schwarz,
R.R.Mendel,
and
M.W.Ribbe
(2009).
Molybdenum cofactors, enzymes and pathways.
|
| |
Nature,
460,
839-847.
|
 |
|
|
|
|
 |
J.B.Glass,
F.Wolfe-Simon,
and
A.D.Anbar
(2009).
Coevolution of metal availability and nitrogen assimilation in cyanobacteria and algae.
|
| |
Geobiology,
7,
100-123.
|
 |
|
|
|
|
 |
K.J.Waldron,
and
N.J.Robinson
(2009).
How do bacterial cells ensure that metalloproteins get the correct metal?
|
| |
Nat Rev Microbiol,
7,
25-35.
|
 |
|
|
|
|
 |
R.Hänsch,
and
R.R.Mendel
(2009).
Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl).
|
| |
Curr Opin Plant Biol,
12,
259-266.
|
 |
|
|
|
|
 |
R.R.Mendel
(2009).
Cell biology of molybdenum.
|
| |
Biofactors,
35,
429-434.
|
 |
|
|
|
|
 |
J.R.Andreesen,
and
K.Makdessi
(2008).
Tungsten, the surprisingly positively acting heavy metal element for prokaryotes.
|
| |
Ann N Y Acad Sci,
1125,
215-229.
|
 |
|
|
|
|
 |
S.J.Hall,
A.Hitchcock,
C.S.Butler,
and
D.J.Kelly
(2008).
A Multicopper oxidase (Cj1516) and a CopA homologue (Cj1161) are major components of the copper homeostasis system of Campylobacter jejuni.
|
| |
J Bacteriol,
190,
8075-8085.
|
 |
|
|
|
|
 |
A.Llamas,
M.Tejada-Jimenez,
D.González-Ballester,
J.J.Higuera,
G.Schwarz,
A.Galván,
and
E.Fernández
(2007).
Chlamydomonas reinhardtii CNX1E reconstitutes molybdenum cofactor biosynthesis in Escherichia coli mutants.
|
| |
Eukaryot Cell,
6,
1063-1067.
|
 |
|
|
|
|
 |
C.Feng,
G.Tollin,
and
J.H.Enemark
(2007).
Sulfite oxidizing enzymes.
|
| |
Biochim Biophys Acta,
1774,
527-539.
|
 |
|
|
|
|
 |
J.D.Nichols,
S.Xiang,
H.Schindelin,
and
K.V.Rajagopalan
(2007).
Mutational analysis of Escherichia coli MoeA: two functional activities map to the active site cleft.
|
| |
Biochemistry,
46,
78-86.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Neumann,
W.Stöcklein,
and
S.Leimkühler
(2007).
Transfer of the molybdenum cofactor synthesized by Rhodobacter capsulatus MoeA to XdhC and MobA.
|
| |
J Biol Chem,
282,
28493-28500.
|
 |
|
|
|
|
 |
M.S.Morrison,
P.A.Cobine,
and
E.L.Hegg
(2007).
Probing the role of copper in the biosynthesis of the molybdenum cofactor in Escherichia coli and Rhodobacter sphaeroides.
|
| |
J Biol Inorg Chem,
12,
1129-1139.
|
 |
|
|
|
|
 |
R.R.Mendel,
A.G.Smith,
A.Marquet,
and
M.J.Warren
(2007).
Metal and cofactor insertion.
|
| |
Nat Prod Rep,
24,
963-971.
|
 |
|
|
|
|
 |
S.Puig,
N.Andrés-Colás,
A.García-Molina,
and
L.Peñarrubia
(2007).
Copper and iron homeostasis in Arabidopsis: responses to metal deficiencies, interactions and biotechnological applications.
|
| |
Plant Cell Environ,
30,
271-290.
|
 |
|
|
|
|
 |
A.Llamas,
T.Otte,
G.Multhaup,
R.R.Mendel,
and
G.Schwarz
(2006).
The Mechanism of nucleotide-assisted molybdenum insertion into molybdopterin. A novel route toward metal cofactor assembly.
|
| |
J Biol Chem,
281,
18343-18350.
|
 |
|
|
|
|
 |
A.Vergnes,
J.Pommier,
R.Toci,
F.Blasco,
G.Giordano,
and
A.Magalon
(2006).
NarJ chaperone binds on two distinct sites of the aponitrate reductase of Escherichia coli to coordinate molybdenum cofactor insertion and assembly.
|
| |
J Biol Chem,
281,
2170-2176.
|
 |
|
|
|
|
 |
G.Schwarz,
and
R.R.Mendel
(2006).
Molybdenum cofactor biosynthesis and molybdenum enzymes.
|
| |
Annu Rev Plant Biol,
57,
623-647.
|
 |
|
|
|
|
 |
I.Paarmann,
B.Schmitt,
B.Meyer,
M.Karas,
and
H.Betz
(2006).
Mass spectrometric analysis of glycine receptor-associated gephyrin splice variants.
|
| |
J Biol Chem,
281,
34918-34925.
|
 |
|
|
|
|
 |
M.L.Schlief,
T.West,
A.M.Craig,
D.M.Holtzman,
and
J.D.Gitlin
(2006).
Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity.
|
| |
Proc Natl Acad Sci U S A,
103,
14919-14924.
|
 |
|
|
|
|
 |
M.Pilon,
S.E.Abdel-Ghany,
C.M.Cohu,
K.A.Gogolin,
and
H.Ye
(2006).
Copper cofactor delivery in plant cells.
|
| |
Curr Opin Plant Biol,
9,
256-263.
|
 |
|
|
|
|
 |
A.F.Peacock,
H.D.Batey,
C.Raendler,
A.C.Whitwood,
R.N.Perutz,
and
A.K.Duhme-Klair
(2005).
A metal-based lumophore tailored to sense biologically relevant oxometalates.
|
| |
Angew Chem Int Ed Engl,
44,
1712-1714.
|
 |
|
|
|
|
 |
J.D.Nichols,
and
K.V.Rajagopalan
(2005).
In vitro molybdenum ligation to molybdopterin using purified components.
|
| |
J Biol Chem,
280,
7817-7822.
|
 |
|
|
|
|
 |
X.Dai,
K.Hayashi,
H.Nozaki,
Y.Cheng,
and
Y.Zhao
(2005).
Genetic and chemical analyses of the action mechanisms of sirtinol in Arabidopsis.
|
| |
Proc Natl Acad Sci U S A,
102,
3129-3134.
|
 |
|
|
|
|
 |
W.N.Hunter
(2004).
Biological chemistry: the making of Moco.
|
| |
Nature,
430,
736-737.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

