 |
PDBsum entry 1uml

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.5.4.4
- adenosine deaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
adenosine + H2O + H+ = inosine + NH4+
|
|
2.
|
2'-deoxyadenosine + H2O + H+ = 2'-deoxyinosine + NH4+
|
|
 |
 |
 |
 |
 |
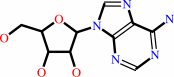 adenosine
adenosine
|
+
|
H2O
|
+
|
H(+)
|
=
|
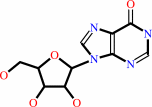 inosine
inosine
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
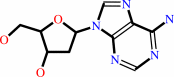 2'-deoxyadenosine
2'-deoxyadenosine
|
+
|
H2O
|
+
|
H(+)
|
=
|
2'-deoxyinosine
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
47:3730-3743
(2004)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure-based design, synthesis, and structure-activity relationship studies of novel non-nucleoside adenosine deaminase inhibitors.
|
|
T.Terasaka,
T.Kinoshita,
M.Kuno,
N.Seki,
K.Tanaka,
I.Nakanishi.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
We disclose herein optimization efforts around the novel, highly potent
non-nucleoside adenosine deaminase (ADA) inhibitor,
1-[(R)-1-hydroxy-4-(6-(3-(1-methylbenzimidazol-2-yl)propionylamino)indol-1-yl)-2-butyl]imidazole-4-carboxamide
1 (K(i)= 7.7 nM), which we recently reported. Structure-based drug design (SBDD)
utilizing the crystal structure of the 1/ADA complex was performed in order to
obtain structure-activity relationships (SAR) for this type of compound
rationally and effectively. To utilize the newly formed hydrophobic space (F2),
replacement of the benzimidazole ring of 1 with a n-propyl chain (4b) or a
simple phenyl ring (4c) was tolerated in terms of binding activity, and the
length of the methylene-spacer was shown to be optimal at two or three.
Replacement of an amide with an ether as a linker was also well tolerated in
terms of binding activity and moreover improved the oral absorption (6a and 6b).
Finally, transformation of indol-1-yl to indol-3-yl resulted in discovery of a
novel highly potent and orally bioavailable ADA inhibitor,
1-[(R)-4-(5-(3-(4-chlorophenyl)propoxy)-1-methylindol-3-yl)-1-hydroxy-2-butyl]imidazole-4-carboxamide
8c.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.P.Yesudas,
F.B.Sayyed,
and
C.H.Suresh
(2011).
Analysis of structural water and CH···π interactions in HIV-1 protease and PTP1B complexes using a hydrogen bond prediction tool, HBPredicT.
|
| |
J Mol Model,
17,
401-413.
|
 |
|
|
|
|
 |
W.C.Van Voorhis,
W.G.Hol,
P.J.Myler,
and
L.J.Stewart
(2009).
The role of medical structural genomics in discovering new drugs for infectious diseases.
|
| |
PLoS Comput Biol,
5,
e1000530.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.T.Larson,
W.Deng,
B.E.Krumm,
A.Napuli,
N.Mueller,
W.C.Van Voorhis,
F.S.Buckner,
E.Fan,
A.Lauricella,
G.DeTitta,
J.Luft,
F.Zucker,
W.G.Hol,
C.L.Verlinde,
and
E.A.Merritt
(2008).
Structures of substrate- and inhibitor-bound adenosine deaminase from a human malaria parasite show a dramatic conformational change and shed light on drug selectivity.
|
| |
J Mol Biol,
381,
975-988.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
G.Koczyk,
L.S.Wyrwicz,
and
L.Rychlewski
(2007).
LigProf: a simple tool for in silico prediction of ligand-binding sites.
|
| |
J Mol Model,
13,
445-455.
|
 |
|
|
|
|
 |
S.P.Williams,
L.F.Kuyper,
and
K.H.Pearce
(2005).
Recent applications of protein crystallography and structure-guided drug design.
|
| |
Curr Opin Chem Biol,
9,
371-380.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

