 |
PDBsum entry 1uby

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.5.1.1
- dimethylallyltranstransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Terpenoid biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
isopentenyl diphosphate + dimethylallyl diphosphate = (2E)- geranyl diphosphate + diphosphate
|
 |
 |
 |
 |
 |
isopentenyl diphosphate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
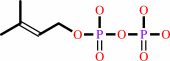 dimethylallyl diphosphate
dimethylallyl diphosphate
|
=
|
(2E)- geranyl diphosphate
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.5.1.10
- (2E,6E)-farnesyl diphosphate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
isopentenyl diphosphate + (2E)-geranyl diphosphate = (2E,6E)-farnesyl diphosphate + diphosphate
|
 |
 |
 |
 |
 |
 isopentenyl diphosphate
isopentenyl diphosphate
|
+
|
(2E)-geranyl diphosphate
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
 (2E,6E)-farnesyl diphosphate
(2E,6E)-farnesyl diphosphate
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Proc Natl Acad Sci U S A
93:15018-15023
(1996)
|
|
PubMed id:
|
|
|
|

|
| |
|
Regulation of product chain length by isoprenyl diphosphate synthases.
|
|
L.C.Tarshis,
P.J.Proteau,
B.A.Kellogg,
J.C.Sacchettini,
C.D.Poulter.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
An analysis of the x-ray structure of homodimeric avian farnesyl diphosphate
synthase (geranyltransferase, EC 2.5.1.10) coupled with information about
conserved amino acids obtained from a sequence alignment of 35 isoprenyl
diphosphate synthases that synthesize farnesyl (C15), geranylgeranyl (C20), and
higher chain length isoprenoid diphosphates suggested that the side chains of
residues corresponding to F112 and F113 in the avian enzyme were important for
determining the ultimate length of the hydrocarbon chains. This hypothesis was
supported by site-directed mutagenesis to transform wild-type avian farnesyl
diphosphate synthase (FPS) into synthases capable of producing geranylgeranyl
diphosphate (F112A), geranylfarnesyl (C25) diphosphate (F113S), and longer chain
prenyl diphosphates (F112A/F113S). An x-ray analysis of the structure of the
F112A/F113S mutant in the apo state and with allylic substrates bound produced
the strongest evidence that these mutations caused the observed change in
product specificity by directly altering the size of the binding pocket for the
growing isoprenoid chain in the active site of the enzyme. The proposed binding
pocket in the apo mutant structure was increased in depth by 5.8 A as compared
with that for the wild-type enzyme. Allylic diphosphates were observed in the
holo structures, bound through magnesium ions to the aspartates of the first of
two conserved aspartate-rich sequences (D117-D121), with the hydrocarbon tails
of all the ligands growing down the hydrophobic pocket toward the mutation site.
A model was constructed to show how the growth of a long chain prenyl product
may proceed by creation of a hydrophobic passageway from the FPS active site to
the outside surface of the enzyme.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.D.Artz,
A.K.Wernimont,
J.E.Dunford,
M.Schapira,
A.Dong,
Y.Zhao,
J.Lew,
R.G.Russell,
F.H.Ebetino,
U.Oppermann,
and
R.Hui
(2011).
Molecular characterization of a novel geranylgeranyl pyrophosphate synthase from Plasmodium parasites.
|
| |
J Biol Chem,
286,
3315-3322.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Hojo,
K.Toga,
D.Watanabe,
T.Yamamoto,
and
K.Maekawa
(2011).
High-level expression of the Geranylgeranyl diphosphate synthase gene in the frontal gland of soldiers in Reticulitermes speratus (Isoptera: Rhinotermitidae).
|
| |
Arch Insect Biochem Physiol,
77,
17-31.
|
 |
|
|
|
|
 |
V.S.Rosso,
S.H.Szajnman,
L.Malayil,
M.Galizzi,
S.N.Moreno,
R.Docampo,
and
J.B.Rodriguez
(2011).
Synthesis and biological evaluation of new 2-alkylaminoethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii targeting farnesyl diphosphate synthase.
|
| |
Bioorg Med Chem,
19,
2211-2217.
|
 |
|
|
|
|
 |
R.Cao,
Y.Zhang,
F.M.Mann,
C.Huang,
D.Mukkamala,
M.P.Hudock,
M.E.Mead,
S.Prisic,
K.Wang,
F.Y.Lin,
T.K.Chang,
R.J.Peters,
and
E.Oldfield
(2010).
Diterpene cyclases and the nature of the isoprene fold.
|
| |
Proteins,
78,
2417-2432.
|
 |
|
|
|
|
 |
T.H.Chang,
F.L.Hsieh,
T.P.Ko,
K.H.Teng,
P.H.Liang,
and
A.H.Wang
(2010).
Structure of a heterotetrameric geranyl pyrophosphate synthase from mint (Mentha piperita) reveals intersubunit regulation.
|
| |
Plant Cell,
22,
454-467.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.H.Taban,
C.Tittiger,
G.J.Blomquist,
and
W.H.Welch
(2009).
Isolation and characterization of farnesyl diphosphate synthase from the cotton boll weevil, Anthonomus grandis.
|
| |
Arch Insect Biochem Physiol,
71,
88.
|
 |
|
|
|
|
 |
C.D.Poulter,
and
C.D.Poulter
(2009).
Bioorganic chemistry. A natural reunion of the physical and life sciences.
|
| |
J Org Chem,
74,
2631-2645.
|
 |
|
|
|
|
 |
D.W.Christianson
(2008).
Unearthing the roots of the terpenome.
|
| |
Curr Opin Chem Biol,
12,
141-150.
|
 |
|
|
|
|
 |
J.D.Artz,
J.E.Dunford,
M.J.Arrowood,
A.Dong,
M.Chruszcz,
K.L.Kavanagh,
W.Minor,
R.G.Russell,
F.H.Ebetino,
U.Oppermann,
and
R.Hui
(2008).
Targeting a uniquely nonspecific prenyl synthase with bisphosphonates to combat cryptosporidiosis.
|
| |
Chem Biol,
15,
1296-1306.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Fujikura,
Y.Maki,
N.Ohya,
M.Satoh,
and
T.Koyama
(2008).
Kinetic studies of Micrococcus luteus B-P 26 undecaprenyl diphosphate synthase reaction using 3-desmethyl allylic substrate analogs.
|
| |
Biosci Biotechnol Biochem,
72,
851-855.
|
 |
|
|
|
|
 |
M.Noike,
T.Katagiri,
T.Nakayama,
T.Koyama,
T.Nishino,
and
H.Hemmi
(2008).
The product chain length determination mechanism of type II geranylgeranyl diphosphate synthase requires subunit interaction.
|
| |
FEBS J,
275,
3921-3933.
|
 |
|
|
|
|
 |
R.Cao,
C.K.Chen,
R.T.Guo,
A.H.Wang,
and
E.Oldfield
(2008).
Structures of a potent phenylalkyl bisphosphonate inhibitor bound to farnesyl and geranylgeranyl diphosphate synthases.
|
| |
Proteins,
73,
431-439.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Saiki,
A.L.Lunceford,
Y.Shi,
B.Marbois,
R.King,
J.Pachuski,
M.Kawamukai,
D.L.Gasser,
and
C.F.Clarke
(2008).
Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2.
|
| |
Am J Physiol Renal Physiol,
295,
F1535-F1544.
|
 |
|
|
|
|
 |
S.H.Szajnman,
G.E.García Liñares,
Z.H.Li,
C.Jiang,
M.Galizzi,
E.J.Bontempi,
M.Ferella,
S.N.Moreno,
R.Docampo,
and
J.B.Rodriguez
(2008).
Synthesis and biological evaluation of 2-alkylaminoethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii targeting farnesyl diphosphate synthase.
|
| |
Bioorg Med Chem,
16,
3283-3290.
|
 |
|
|
|
|
 |
W.Schwab,
R.Davidovich-Rikanati,
and
E.Lewinsohn
(2008).
Biosynthesis of plant-derived flavor compounds.
|
| |
Plant J,
54,
712-732.
|
 |
|
|
|
|
 |
Y.L.Zhang,
and
Z.X.Li
(2008).
Two different farnesyl diphosphate synthase genes exist in the genome of the green peach aphid, Myzus persicae.
|
| |
Genome,
51,
501-510.
|
 |
|
|
|
|
 |
Y.Y.Hsiao,
M.F.Jeng,
W.C.Tsai,
Y.C.Chuang,
C.Y.Li,
T.S.Wu,
C.S.Kuoh,
W.H.Chen,
and
H.H.Chen
(2008).
A novel homodimeric geranyl diphosphate synthase from the orchid Phalaenopsis bellina lacking a DD(X)2-4D motif.
|
| |
Plant J,
55,
719-733.
|
 |
|
|
|
|
 |
J.Poznański,
and
A.Szkopinska
(2007).
Precise bacterial polyprenol length control fails in Saccharomyces cerevisiae.
|
| |
Biopolymers,
86,
155-164.
|
 |
|
|
|
|
 |
M.Hojo,
T.Matsumoto,
and
T.Miura
(2007).
Cloning and expression of a geranylgeranyl diphosphate synthase gene: insights into the synthesis of termite defence secretion.
|
| |
Insect Mol Biol,
16,
121-131.
|
 |
|
|
|
|
 |
H.V.Thulasiram,
and
C.D.Poulter
(2006).
Farnesyl diphosphate synthase: the art of compromise between substrate selectivity and stereoselectivity.
|
| |
J Am Chem Soc,
128,
15819-15823.
|
 |
|
|
|
|
 |
J.M.Rondeau,
F.Bitsch,
E.Bourgier,
M.Geiser,
R.Hemmig,
M.Kroemer,
S.Lehmann,
P.Ramage,
S.Rieffel,
A.Strauss,
J.R.Green,
and
W.Jahnke
(2006).
Structural basis for the exceptional in vivo efficacy of bisphosphonate drugs.
|
| |
ChemMedChem,
1,
267-273.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.L.Kavanagh,
K.Guo,
J.E.Dunford,
X.Wu,
S.Knapp,
F.H.Ebetino,
M.J.Rogers,
R.G.Russell,
and
U.Oppermann
(2006).
The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs.
|
| |
Proc Natl Acad Sci U S A,
103,
7829-7834.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Cusson,
C.Béliveau,
S.E.Sen,
S.Vandermoten,
R.G.Rutledge,
D.Stewart,
F.Francis,
E.Haubruge,
P.Rehse,
D.J.Huggins,
A.P.Dowling,
and
G.H.Grant
(2006).
Characterization and tissue-specific expression of two lepidopteran farnesyl diphosphate synthase homologs: implications for the biosynthesis of ethyl-substituted juvenile hormones.
|
| |
Proteins,
65,
742-758.
|
 |
|
|
|
|
 |
R.Schwartz,
and
J.King
(2006).
Frequencies of hydrophobic and hydrophilic runs and alternations in proteins of known structure.
|
| |
Protein Sci,
15,
102-112.
|
 |
|
|
|
|
 |
S.B.Gabelli,
J.S.McLellan,
A.Montalvetti,
E.Oldfield,
R.Docampo,
and
L.M.Amzel
(2006).
Structure and mechanism of the farnesyl diphosphate synthase from Trypanosoma cruzi: implications for drug design.
|
| |
Proteins,
62,
80-88.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Liu,
Q.Li,
and
L.Lai
(2006).
A combinatorial score to distinguish biological and nonbiological protein-protein interfaces.
|
| |
Proteins,
64,
68-78.
|
 |
|
|
|
|
 |
X.Liu,
H.Wu,
J.Ye,
Q.Yuan,
and
H.Zhang
(2006).
Cloning and characterization of the ddsA gene encoding decaprenyl diphosphate synthase from Rhodobacter capsulatus B10.
|
| |
Can J Microbiol,
52,
1141-1147.
|
 |
|
|
|
|
 |
Y.Kharel,
S.Takahashi,
S.Yamashita,
and
T.Koyama
(2006).
Manipulation of prenyl chain length determination mechanism of cis-prenyltransferases.
|
| |
FEBS J,
273,
647-657.
|
 |
|
|
|
|
 |
A.B.Gilg,
J.C.Bearfield,
C.Tittiger,
W.H.Welch,
and
G.J.Blomquist
(2005).
Isolation and functional expression of an animal geranyl diphosphate synthase and its role in bark beetle pheromone biosynthesis.
|
| |
Proc Natl Acad Sci U S A,
102,
9760-9765.
|
 |
|
|
|
|
 |
B.Ku,
J.C.Jeong,
B.N.Mijts,
C.Schmidt-Dannert,
and
J.S.Dordick
(2005).
Preparation, characterization, and optimization of an in vitro C30 carotenoid pathway.
|
| |
Appl Environ Microbiol,
71,
6578-6583.
|
 |
|
|
|
|
 |
D.Umeno,
A.V.Tobias,
and
F.H.Arnold
(2005).
Diversifying carotenoid biosynthetic pathways by directed evolution.
|
| |
Microbiol Mol Biol Rev,
69,
51-78.
|
 |
|
|
|
|
 |
F.Bouvier,
A.Rahier,
and
B.Camara
(2005).
Biogenesis, molecular regulation and function of plant isoprenoids.
|
| |
Prog Lipid Res,
44,
357-429.
|
 |
|
|
|
|
 |
H.Y.Sun,
T.P.Ko,
C.J.Kuo,
R.T.Guo,
C.C.Chou,
P.H.Liang,
and
A.H.Wang
(2005).
Homodimeric hexaprenyl pyrophosphate synthase from the thermoacidophilic crenarchaeon Sulfolobus solfataricus displays asymmetric subunit structures.
|
| |
J Bacteriol,
187,
8137-8148.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.H.Choi,
Y.W.Ryu,
and
J.H.Seo
(2005).
Biotechnological production and applications of coenzyme Q10.
|
| |
Appl Microbiol Biotechnol,
68,
9.
|
 |
|
|
|
|
 |
P.B.Crowley,
and
A.Golovin
(2005).
Cation-pi interactions in protein-protein interfaces.
|
| |
Proteins,
59,
231-239.
|
 |
|
|
|
|
 |
D.Mekkriengkrai,
T.Sando,
K.Hirooka,
J.Sakdapipanich,
Y.Tanaka,
E.Fukusaki,
and
A.Kobayashi
(2004).
Cloning and characterization of farnesyl diphosphate synthase from the rubber-producing mushroom Lactarius chrysorrheus.
|
| |
Biosci Biotechnol Biochem,
68,
2360-2368.
|
 |
|
|
|
|
 |
J.K.Lee,
G.Her,
S.Y.Kim,
and
J.H.Seo
(2004).
Cloning and functional expression of the dps gene encoding decaprenyl diphosphate synthase from Agrobacterium tumefaciens.
|
| |
Biotechnol Prog,
20,
51-56.
|
 |
|
|
|
|
 |
J.Mao,
Y.G.Gao,
S.Odeh,
H.Robinson,
A.Montalvetti,
R.Docampo,
and
E.Oldfield
(2004).
Crystallization and preliminary X-ray diffraction study of the farnesyl diphosphate synthase from Trypanosoma brucei.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
1863-1866.
|
 |
|
|
|
|
 |
V.E.Ahn,
E.I.Lo,
C.K.Engel,
L.Chen,
P.M.Hwang,
L.E.Kay,
R.E.Bishop,
and
G.G.Privé
(2004).
A hydrocarbon ruler measures palmitate in the enzymatic acylation of endotoxin.
|
| |
EMBO J,
23,
2931-2941.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.J.Mau,
S.Garneau,
A.A.Scholte,
J.E.Van Fleet,
J.C.Vederas,
and
K.Cornish
(2003).
Protein farnesyltransferase inhibitors interfere with farnesyl diphosphate binding by rubber transferase.
|
| |
Eur J Biochem,
270,
3939-3945.
|
 |
|
|
|
|
 |
H.Hemmi,
M.Noike,
T.Nakayama,
and
T.Nishino
(2003).
An alternative mechanism of product chain-length determination in type III geranylgeranyl diphosphate synthase.
|
| |
Eur J Biochem,
270,
2186-2194.
|
 |
|
|
|
|
 |
D.Umeno,
A.V.Tobias,
and
F.H.Arnold
(2002).
Evolution of the C30 carotenoid synthase CrtM for function in a C40 pathway.
|
| |
J Bacteriol,
184,
6690-6699.
|
 |
|
|
|
|
 |
E.Shaw,
and
J.S.Dordick
(2002).
Predicting amino acid residues responsible for enzyme specificity solely from protein sequences.
|
| |
Biotechnol Bioeng,
79,
295-300.
|
 |
|
|
|
|
 |
H.C.Schmid,
V.Rassadina,
U.Oster,
S.Schoch,
and
W.Rüdiger
(2002).
Pre-loading of chlorophyll synthase with tetraprenyl diphosphate is an obligatory step in chlorophyll biosynthesis.
|
| |
Biol Chem,
383,
1769-1778.
|
 |
|
|
|
|
 |
H.Hemmi,
S.Ikejiri,
S.Yamashita,
and
T.Nishino
(2002).
Novel medium-chain prenyl diphosphate synthase from the thermoacidophilic archaeon Sulfolobus solfataricus.
|
| |
J Bacteriol,
184,
615-620.
|
 |
|
|
|
|
 |
M.J.Rynkiewicz,
D.E.Cane,
and
D.W.Christianson
(2002).
X-ray crystal structures of D100E trichodiene synthase and its pyrophosphate complex reveal the basis for terpene product diversity.
|
| |
Biochemistry,
41,
1732-1741.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.H.Liang,
T.P.Ko,
and
A.H.Wang
(2002).
Structure, mechanism and function of prenyltransferases.
|
| |
Eur J Biochem,
269,
3339-3354.
|
 |
|
|
|
|
 |
H.C.Schmid,
U.Oster,
J.Kögel,
S.Lenz,
and
W.Rüdiger
(2001).
Cloning and characterisation of chlorophyll synthase from Avena sativa.
|
| |
Biol Chem,
382,
903-911.
|
 |
|
|
|
|
 |
M.Fujihashi,
Y.W.Zhang,
Y.Higuchi,
X.Y.Li,
T.Koyama,
and
K.Miki
(2001).
Crystal structure of cis-prenyl chain elongating enzyme, undecaprenyl diphosphate synthase.
|
| |
Proc Natl Acad Sci U S A,
98,
4337-4342.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.J.Rynkiewicz,
D.E.Cane,
and
D.W.Christianson
(2001).
Structure of trichodiene synthase from Fusarium sporotrichioides provides mechanistic inferences on the terpene cyclization cascade.
|
| |
Proc Natl Acad Sci U S A,
98,
13543-13548.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Barkovich,
and
J.C.Liao
(2001).
Metabolic engineering of isoprenoids.
|
| |
Metab Eng,
3,
27-39.
|
 |
|
|
|
|
 |
T.Soderberg,
and
C.D.Poulter
(2001).
Escherichia coli dimethylallyl diphosphate:tRNA dimethylallyltransferase: site-directed mutagenesis of highly conserved residues.
|
| |
Biochemistry,
40,
1734-1740.
|
 |
|
|
|
|
 |
W.Sitthithaworn,
N.Kojima,
E.Viroonchatapan,
D.Y.Suh,
N.Iwanami,
T.Hayashi,
M.Noji,
K.Saito,
Y.Niwa,
and
U.Sankawa
(2001).
Geranylgeranyl diphosphate synthase from Scoparia dulcis and Croton sublyratus. Plastid localization and conversion to a farnesyl diphosphate synthase by mutagenesis.
|
| |
Chem Pharm Bull (Tokyo),
49,
197-202.
|
 |
|
|
|
|
 |
A.Tachibana,
Y.Yano,
S.Otani,
N.Nomura,
Y.Sako,
and
M.Taniguchi
(2000).
Novel prenyltransferase gene encoding farnesylgeranyl diphosphate synthase from a hyperthermophilic archaeon, Aeropyrum pernix. Molecularevolution with alteration in product specificity.
|
| |
Eur J Biochem,
267,
321-328.
|
 |
|
|
|
|
 |
D.Vicent,
E.Maratos-Flier,
and
C.R.Kahn
(2000).
The branch point enzyme of the mevalonate pathway for protein prenylation is overexpressed in the ob/ob mouse and induced by adipogenesis.
|
| |
Mol Cell Biol,
20,
2158-2166.
|
 |
|
|
|
|
 |
J.E.Grove,
R.J.Brown,
and
D.J.Watts
(2000).
The intracellular target for the antiresorptive aminobisphosphonate drugs in Dictyostelium discoideum is the enzyme farnesyl diphosphate synthase.
|
| |
J Bone Miner Res,
15,
971-981.
|
 |
|
|
|
|
 |
J.J.Pan,
L.W.Yang,
and
P.H.Liang
(2000).
Effect of site-directed mutagenesis of the conserved aspartate and glutamate on E. coli undecaprenyl pyrophosphate synthase catalysis.
|
| |
Biochemistry,
39,
13856-13861.
|
 |
|
|
|
|
 |
K.Hirooka,
S.Ohnuma,
A.Koike-Takeshita,
T.Koyama,
and
T.Nishino
(2000).
Mechanism of product chain length determination for heptaprenyl diphosphate synthase from Bacillus stearothermophilus.
|
| |
Eur J Biochem,
267,
4520-4528.
|
 |
|
|
|
|
 |
S.M.Stanley Fernandez,
B.A.Kellogg,
and
C.D.Poulter
(2000).
Farnesyl diphosphate synthase. Altering the catalytic site to select for geranyl diphosphate activity.
|
| |
Biochemistry,
39,
15316-15321.
|
 |
|
|
|
|
 |
T.Koyama,
Y.Gotoh,
and
T.Nishino
(2000).
Intersubunit location of the active site of farnesyl diphosphate synthase: reconstruction of active enzymes by hybrid-type heteromeric dimers of site-directed mutants.
|
| |
Biochemistry,
39,
463-469.
|
 |
|
|
|
|
 |
T.R.Tansey,
and
I.Shechter
(2000).
Structure and regulation of mammalian squalene synthase.
|
| |
Biochim Biophys Acta,
1529,
49-62.
|
 |
|
|
|
|
 |
C.C.Burke,
M.R.Wildung,
and
R.Croteau
(1999).
Geranyl diphosphate synthase: cloning, expression, and characterization of this prenyltransferase as a heterodimer.
|
| |
Proc Natl Acad Sci U S A,
96,
13062-13067.
|
 |
|
|
|
|
 |
C.M.Apfel,
B.Takács,
M.Fountoulakis,
M.Stieger,
and
W.Keck
(1999).
Use of genomics to identify bacterial undecaprenyl pyrophosphate synthetase: cloning, expression, and characterization of the essential uppS gene.
|
| |
J Bacteriol,
181,
483-492.
|
 |
|
|
|
|
 |
C.Ohto,
C.Ishida,
A.Koike-Takeshita,
K.Yokoyama,
M.Muramatsu,
T.Nishino,
and
S.Obata
(1999).
Gene cloning and overexpression of a geranylgeranyl diphosphate synthase of an extremely thermophilic bacterium, Thermus thermophilus.
|
| |
Biosci Biotechnol Biochem,
63,
261-270.
|
 |
|
|
|
|
 |
H.J.Chiu,
J.J.Reddick,
T.P.Begley,
and
S.E.Ealick
(1999).
Crystal structure of thiamin phosphate synthase from Bacillus subtilis at 1.25 A resolution.
|
| |
Biochemistry,
38,
6460-6470.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Kim,
and
C.H.Yang
(1999).
Active site determination of yeast geranylgeranyl protein transferase type I expressed in Escherichia coli.
|
| |
Eur J Biochem,
265,
105-111.
|
 |
|
|
|
|
 |
K.Wang,
and
S.Ohnuma
(1999).
Chain-length determination mechanism of isoprenyl diphosphate synthases and implications for molecular evolution.
|
| |
Trends Biochem Sci,
24,
445-451.
|
 |
|
|
|
|
 |
M.Castillo-Gracia,
and
F.Couillaud
(1999).
Molecular cloning and tissue expression of an insect farnesyl diphosphate synthase.
|
| |
Eur J Biochem,
262,
365-370.
|
 |
|
|
|
|
 |
M.Fujihashi,
N.Shimizu,
Y.W.Zhang,
T.Koyama,
and
K.Miki
(1999).
Crystallization and preliminary X-ray diffraction studies of undecaprenyl diphosphate synthase from Micrococcus luteus B-P 26.
|
| |
Acta Crystallogr D Biol Crystallogr,
55,
1606-1607.
|
 |
|
|
|
|
 |
T.Koyama
(1999).
Molecular analysis of prenyl chain elongating enzymes.
|
| |
Biosci Biotechnol Biochem,
63,
1671-1676.
|
 |
|
|
|
|
 |
U.Müh,
A.J.Sinskey,
D.P.Kirby,
W.S.Lane,
and
J.Stubbe
(1999).
PHA synthase from chromatium vinosum: cysteine 149 is involved in covalent catalysis.
|
| |
Biochemistry,
38,
826-837.
|
 |
|
|
|
|
 |
Y.W.Zhang,
X.Y.Li,
H.Sugawara,
and
T.Koyama
(1999).
Site-directed mutagenesis of the conserved residues in component I of Bacillus subtilis heptaprenyl diphosphate synthase.
|
| |
Biochemistry,
38,
14638-14643.
|
 |
|
|
|
|
 |
F.X.Cunningham,
and
E.Gantt
(1998).
GENES AND ENZYMES OF CAROTENOID BIOSYNTHESIS IN PLANTS.
|
| |
Annu Rev Plant Physiol Plant Mol Biol,
49,
557-583.
|
 |
|
|
|
|
 |
K.U.Wendt,
and
G.E.Schulz
(1998).
Isoprenoid biosynthesis: manifold chemistry catalyzed by similar enzymes.
|
| |
Structure,
6,
127-133.
|
 |
|
|
|
|
 |
S.B.Long,
P.J.Casey,
and
L.S.Beese
(1998).
Cocrystal structure of protein farnesyltransferase complexed with a farnesyl diphosphate substrate.
|
| |
Biochemistry,
37,
9612-9618.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.A.Kellogg,
and
C.D.Poulter
(1997).
Chain elongation in the isoprenoid biosynthetic pathway.
|
| |
Curr Opin Chem Biol,
1,
570-578.
|
 |
|
|
|
|
 |
H.W.Park,
and
L.S.Beese
(1997).
Protein farnesyltransferase.
|
| |
Curr Opin Struct Biol,
7,
873-880.
|
 |
|
|
|
|
 |
J.M.Dolence,
D.B.Rozema,
and
C.D.Poulter
(1997).
Yeast protein farnesyltransferase. Site-directed mutagenesis of conserved residues in the beta-subunit.
|
| |
Biochemistry,
36,
9246-9252.
|
 |
|
|
|
|
 |
K.Okada,
Y.Kamiya,
X.Zhu,
K.Suzuki,
K.Tanaka,
T.Nakagawa,
H.Matsuda,
and
M.Kawamukai
(1997).
Cloning of the sdsA gene encoding solanesyl diphosphate synthase from Rhodobacter capsulatus and its functional expression in Escherichia coli and Saccharomyces cerevisiae.
|
| |
J Bacteriol,
179,
5992-5998.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

