
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.4.2.1.20
- tryptophan synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Tryptophan Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate + L-serine = D-glyceraldehyde 3-phosphate + L-tryptophan + H2O
|
 |
 |
 |
 |
 |
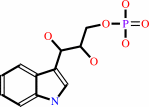 (1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate
(1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate
|
+
|
 L-serine
L-serine
|
=
|
 D-glyceraldehyde 3-phosphate
D-glyceraldehyde 3-phosphate
|
+
|
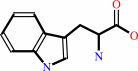 L-tryptophan
L-tryptophan
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
35:4211-4221
(1996)
|
|
PubMed id:
|
|
|
|

|
| |
|
Exchange of K+ or Cs+ for Na+ induces local and long-range changes in the three-dimensional structure of the tryptophan synthase alpha2beta2 complex.
|
|
S.Rhee,
K.D.Parris,
S.A.Ahmed,
E.W.Miles,
D.R.Davies.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Monovalent cations activate the pyridoxal phosphate-dependent reactions of
tryptophan synthase and affect intersubunit communication in the alpha2beta2
complex. We report refined crystal structures of the tryptophan synthase
alpha2beta2 complex from Salmonella typhimurium in the presence of K+ at 2.0
angstrom and of Cs+ at 2.3 angstrom. Comparison of these structures with the
recently refined structure in the presence of Na+ shows that each monovalent
cation binds at approximately the same position about 8 angstrom from the
phosphate of pyridoxal phosphate. Na+ and K+ are coordinated to the carbonyl
oxygens of beta Phe-306, beta Ser-308, and beta Gly-232 and to two or one water
molecule, respectively. Cs+ is coordinated to the carbonyl oxygens of beta
Phe-306, beta Ser-308, beta Gly-232, beta Val-231, beta Gly-268 and beta
Leu-304. A second binding site for Cs+ is located in the beta/beta interface on
the 2-fold axis with four carbonyl oxygens in the coordination sphere. In
addition to local changes in structure close to the cation binding site, a
number of long-range changes are observed. The K+ and Cs+ structures differ from
the Na+ structure with respect to the positions of beta Asp-305, beta Lys-167,
and alpha Asp-56. One unexpected result of this investigation is the movement of
the side chains of beta Phe-280 and beta Tyr-279 from a position partially
blocking the tunnel in the Na+ structure to a position lining the surface of the
tunnel in the K+ and Cs+ structures. The results provide a structural basis for
understanding the effects of cations on activity and intersubunit communication.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.Nishio,
K.Ogasahara,
Y.Morimoto,
T.Tsukihara,
S.J.Lee,
and
K.Yutani
(2010).
Large conformational changes in the Escherichia coli tryptophan synthase beta(2) subunit upon pyridoxal 5'-phosphate binding.
|
| |
FEBS J,
277,
2157-2170.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Raboni,
S.Bettati,
and
A.Mozzarelli
(2009).
Tryptophan synthase: a mine for enzymologists.
|
| |
Cell Mol Life Sci,
66,
2391-2403.
|
 |
|
|
|
|
 |
T.R.Barends,
T.Domratcheva,
V.Kulik,
L.Blumenstein,
D.Niks,
M.F.Dunn,
and
I.Schlichting
(2008).
Structure and mechanistic implications of a tryptophan synthase quinonoid intermediate.
|
| |
Chembiochem,
9,
1024-1028.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Mouilleron,
and
B.Golinelli-Pimpaneau
(2007).
Conformational changes in ammonia-channeling glutamine amidotransferases.
|
| |
Curr Opin Struct Biol,
17,
653-664.
|
 |
|
|
|
|
 |
E.Di Cera
(2006).
A structural perspective on enzymes activated by monovalent cations.
|
| |
J Biol Chem,
281,
1305-1308.
|
 |
|
|
|
|
 |
D.Davies,
and
D.Davies
(2005).
A quiet life with proteins.
|
| |
Annu Rev Biophys Biomol Struct,
34,
1.
|
 |
|
|
|
|
 |
A.O.Pineda,
C.J.Carrell,
L.A.Bush,
S.Prasad,
S.Caccia,
Z.W.Chen,
F.S.Mathews,
and
E.Di Cera
(2004).
Molecular dissection of Na+ binding to thrombin.
|
| |
J Biol Chem,
279,
31842-31853.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
F.Schiaretti,
S.Bettati,
C.Viappiani,
and
A.Mozzarelli
(2004).
pH dependence of tryptophan synthase catalytic mechanism: I. The first stage, the beta-elimination reaction.
|
| |
J Biol Chem,
279,
29572-29582.
|
 |
|
|
|
|
 |
Y.Hioki,
K.Ogasahara,
S.J.Lee,
J.Ma,
M.Ishida,
Y.Yamagata,
Y.Matsuura,
M.Ota,
M.Ikeguchi,
S.Kuramitsu,
and
K.Yutani
(2004).
The crystal structure of the tryptophan synthase beta subunit from the hyperthermophile Pyrococcus furiosus. Investigation of stabilization factors.
|
| |
Eur J Biochem,
271,
2624-2635.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Osborne,
Q.Teng,
E.W.Miles,
and
R.S.Phillips
(2003).
Detection of open and closed conformations of tryptophan synthase by 15N-heteronuclear single-quantum coherence nuclear magnetic resonance of bound 1-15N-L-tryptophan.
|
| |
J Biol Chem,
278,
44083-44090.
|
 |
|
|
|
|
 |
R.Amaro,
E.Tajkhorshid,
and
Z.Luthey-Schulten
(2003).
Developing an energy landscape for the novel function of a (beta/alpha)8 barrel: ammonia conduction through HisF.
|
| |
Proc Natl Acad Sci U S A,
100,
7599-7604.
|
 |
|
|
|
|
 |
S.Prasad,
K.J.Wright,
D.Banerjee Roy,
L.A.Bush,
A.M.Cantwell,
and
E.Di Cera
(2003).
Redesigning the monovalent cation specificity of an enzyme.
|
| |
Proc Natl Acad Sci U S A,
100,
13785-13790.
|
 |
|
|
|
|
 |
M.Weyand,
I.Schlichting,
P.Herde,
A.Marabotti,
and
A.Mozzarelli
(2002).
Crystal structure of the beta Ser178--> Pro mutant of tryptophan synthase. A "knock-out" allosteric enzyme.
|
| |
J Biol Chem,
277,
10653-10660.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
O.Hur,
D.Niks,
P.Casino,
and
M.F.Dunn
(2002).
Proton transfers in the beta-reaction catalyzed by tryptophan synthase.
|
| |
Biochemistry,
41,
9991.
|
 |
|
|
|
|
 |
R.M.Harris,
and
M.F.Dunn
(2002).
Intermediate trapping via a conformational switch in the Na(+)-activated tryptophan synthase bienzyme complex.
|
| |
Biochemistry,
41,
9982-9990.
|
 |
|
|
|
|
 |
E.W.Miles
(2001).
Tryptophan synthase: a multienzyme complex with an intramolecular tunnel.
|
| |
Chem Rec,
1,
140-151.
|
 |
|
|
|
|
 |
X.Huang,
H.M.Holden,
and
F.M.Raushel
(2001).
Channeling of substrates and intermediates in enzyme-catalyzed reactions.
|
| |
Annu Rev Biochem,
70,
149-180.
|
 |
|
|
|
|
 |
A.Mozzarelli,
A.Peracchi,
B.Rovegno,
G.Dalè,
G.L.Rossi,
and
M.F.Dunn
(2000).
Effect of pH and monovalent cations on the formation of quinonoid intermediates of the tryptophan synthase alpha(2)beta(2) complex in solution and in the crystal.
|
| |
J Biol Chem,
275,
6956-6962.
|
 |
|
|
|
|
 |
D.Kohls,
T.Sulea,
E.O.Purisima,
R.E.MacKenzie,
and
A.Vrielink
(2000).
The crystal structure of the formiminotransferase domain of formiminotransferase-cyclodeaminase: implications for substrate channeling in a bifunctional enzyme.
|
| |
Structure,
8,
35-46.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
P.Rondard,
and
H.Bedouelle
(2000).
Mutational scanning of a hairpin loop in the tryptophan synthase beta-subunit implicated in allostery and substrate channeling.
|
| |
Biol Chem,
381,
1185-1193.
|
 |
|
|
|
|
 |
E.W.Miles,
S.Rhee,
and
D.R.Davies
(1999).
The molecular basis of substrate channeling.
|
| |
J Biol Chem,
274,
12193-12196.
|
 |
|
|
|
|
 |
H.S.Ro,
and
E.Wilson Miles
(1999).
Catalytic mechanism of the tryptophan synthase alpha(2)beta(2) complex. Effects of pH, isotopic substitution, and allosteric ligands.
|
| |
J Biol Chem,
274,
31189-31194.
|
 |
|
|
|
|
 |
K.D.Schnackerz,
C.H.Tai,
R.K.Pötsch,
and
P.F.Cook
(1999).
Substitution of pyridoxal 5'-phosphate in D-serine dehydratase from Escherichia coli by cofactor analogues provides information on cofactor binding and catalysis.
|
| |
J Biol Chem,
274,
36935-36943.
|
 |
|
|
|
|
 |
D.T.Gallagher,
G.L.Gilliland,
G.Xiao,
J.Zondlo,
K.E.Fisher,
D.Chinchilla,
and
E.Eisenstein
(1998).
Structure and control of pyridoxal phosphate dependent allosteric threonine deaminase.
|
| |
Structure,
6,
465-475.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Davoodi,
P.M.Drown,
R.K.Bledsoe,
R.Wallin,
G.D.Reinhart,
and
S.M.Hutson
(1998).
Overexpression and characterization of the human mitochondrial and cytosolic branched-chain aminotransferases.
|
| |
J Biol Chem,
273,
4982-4989.
|
 |
|
|
|
|
 |
J.L.Smith
(1998).
Glutamine PRPP amidotransferase: snapshots of an enzyme in action.
|
| |
Curr Opin Struct Biol,
8,
686-694.
|
 |
|
|
|
|
 |
J.N.Jansonius
(1998).
Structure, evolution and action of vitamin B6-dependent enzymes.
|
| |
Curr Opin Struct Biol,
8,
759-769.
|
 |
|
|
|
|
 |
K.D.Schnackerz,
and
A.Mozzarelli
(1998).
Plasticity of the tryptophan synthase active site probed by 31P NMR spectroscopy.
|
| |
J Biol Chem,
273,
33247-33253.
|
 |
|
|
|
|
 |
P.Rondard,
and
H.Bedouelle
(1998).
A mutational approach shows similar mechanisms of recognition for the isolated and integrated versions of a protein epitope.
|
| |
J Biol Chem,
273,
34753-34759.
|
 |
|
|
|
|
 |
S.Rhee,
E.W.Miles,
and
D.R.Davies
(1998).
Cryo-crystallography of a true substrate, indole-3-glycerol phosphate, bound to a mutant (alphaD60N) tryptophan synthase alpha2beta2 complex reveals the correct orientation of active site alphaGlu49.
|
| |
J Biol Chem,
273,
8553-8555.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.Madern,
and
G.Zaccai
(1997).
Stabilisation of halophilic malate dehydrogenase from Haloarcula marismortui by divalent cations -- effects of temperature, water isotope, cofactor and pH.
|
| |
Eur J Biochem,
249,
607-611.
|
 |
|
|
|
|
 |
L.Ramírez-Silva,
J.Oria,
A.Gómez-Puyou,
and
M.Tuena de Gómez-Puyou
(1997).
The contribution of water to the selectivity of pyruvate kinase for Na+ and K+.
|
| |
Eur J Biochem,
250,
583-589.
|
 |
|
|
|
|
 |
P.Pan,
E.Woehl,
and
M.F.Dunn
(1997).
Protein architecture, dynamics and allostery in tryptophan synthase channeling.
|
| |
Trends Biochem Sci,
22,
22-27.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

