 |
PDBsum entry 1pwc

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.4.16.4
- serine-type D-Ala-D-Ala carboxypeptidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
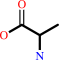
D-alanyl-D-alanine + H2O = 2 D-alanine
|
 |
 |
 |
 |
 |
Bound ligand (Het Group name = )
matches with 41.00% similarity
|
+
|
|
=
|
 2
×
2
×
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
345:521-533
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of complexes between the R61 DD-peptidase and peptidoglycan-mimetic beta-lactams: a non-covalent complex with a "perfect penicillin".
|
|
N.R.Silvaggi,
H.R.Josephine,
A.P.Kuzin,
R.Nagarajan,
R.F.Pratt,
J.A.Kelly.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The bacterial D-alanyl-D-alanine transpeptidases (DD-peptidases) are the killing
targets of beta-lactams, the most important clinical defense against bacterial
infections. However, due to the constant development of antibiotic-resistance
mechanisms by bacteria, there is an ever-present need for new, more effective
antimicrobial drugs. While enormous numbers of beta-lactam compounds have been
tested for antibiotic activity in over 50 years of research, the success of a
beta-lactam structure in terms of antibiotic activity remains unpredictable.
Tipper and Strominger suggested long ago that beta-lactams inhibit DD-peptidases
because they mimic the D-alanyl-D-alanine motif of the peptidoglycan substrate
of these enzymes. They also predicted that beta-lactams having a
peptidoglycan-mimetic side-chain might be better antibiotics than their
non-specific counterparts, but decades of research have not provided any
evidence for this. We have recently described two such novel beta-lactams. The
first is a penicillin having the glycyl-L-alpha-amino-epsilon-pimelyl side-chain
of Streptomyces strain R61 peptidoglycan, making it the "perfect
penicillin" for this organism. The other is a cephalosporin with the same
side-chain. Here, we describe the X-ray crystal structures of the perfect
penicillin in non-covalent and covalent complexes with the Streptomyces R61
DD-peptidase. The structure of the non-covalent enzyme-inhibitor complex is the
first such complex to be trapped crystallographically with a DD-peptidase. In
addition, the covalent complex of the peptidyl-cephalosporin with the R61
DD-peptidase is described. Finally, two covalent complexes with the traditional
beta-lactams benzylpenicillin and cephalosporin C were determined for comparison
with the peptidyl beta-lactams. These structures, together with relevant
kinetics data, support Tipper and Strominger's assertion that
peptidoglycan-mimetic side-chains should improve beta-lactams as inhibitors of
DD-peptidases.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Representations of the active sites in the five
complex structures (E2 (a), E2* (b), E3* (c), E4* (d), and E5*
(e)) showing the interactions between active-site functional
groups and the inhibitors (broken lines). Distances (in Å)
for the interactions are denoted adjacent to the broken lines.
Covalent cross-links between Lys65, His108, and Tyr159 (see
Materials and Methods) are shown in (a).
|
 |
Figure 4.
Figure 4. Stereo view showing a superposition of the active
sites of the E2 complex (a), and E2* complex (b), onto the ES
complex. Carbon atoms of the active-site residues in the ES
complex are colored grey, while those in the tetrapeptide
substrate are colored black. The E2, E2*, and model tetrahedral
intermediate complexes are shown with yellow carbon atoms in the
protein residues and orange in compound 2. Notice the similarity
between the conformations of the substrate and inhibitor,
especially the region near the catalytic Ser62. The rmsd between
E2 and ES is 0.22 Å, while that of the E2* and ES
structures is 0.31 Å, for all atoms in the 16 active-site
residues. In (c) the E2 structure is overlaid on an
energy-minimized model of the tetrahedral intermediate with
peptide substrate. The Figure was produced using MOLSCRIPT.39
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2005,
345,
521-533)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.F.Kluge,
and
R.C.Petter
(2010).
Acylating drugs: redesigning natural covalent inhibitors.
|
| |
Curr Opin Chem Biol,
14,
421-427.
|
 |
|
|
|
|
 |
E.Sauvage,
A.J.Powell,
J.Heilemann,
H.R.Josephine,
P.Charlier,
C.Davies,
and
R.F.Pratt
(2008).
Crystal structures of complexes of bacterial DD-peptidases with peptidoglycan-mimetic ligands: the substrate specificity puzzle.
|
| |
J Mol Biol,
381,
383-393.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Sauvage,
F.Kerff,
M.Terrak,
J.A.Ayala,
and
P.Charlier
(2008).
The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis.
|
| |
FEMS Microbiol Rev,
32,
234-258.
|
 |
|
|
|
|
 |
I.Kumar,
H.R.Josephine,
and
R.F.Pratt
(2007).
Reactions of peptidoglycan-mimetic beta-lactams with penicillin-binding proteins in vivo and in membranes.
|
| |
ACS Chem Biol,
2,
620-624.
|
 |
|
|
|
|
 |
S.A.Adediran,
I.Kumar,
and
R.F.Pratt
(2006).
Deacylation transition states of a bacterial DD-peptidase.
|
| |
Biochemistry,
45,
13074-13082.
|
 |
|
|
|
|
 |
E.Sauvage,
R.Herman,
S.Petrella,
C.Duez,
F.Bouillenne,
J.M.Frère,
and
P.Charlier
(2005).
Crystal structure of the Actinomadura R39 DD-peptidase reveals new domains in penicillin-binding proteins.
|
| |
J Biol Chem,
280,
31249-31256.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.S.Wilke,
A.L.Lovering,
and
N.C.Strynadka
(2005).
Beta-lactam antibiotic resistance: a current structural perspective.
|
| |
Curr Opin Microbiol,
8,
525-533.
|
 |
|
|
|
|
 |
N.Rhazi,
M.Delmarcelle,
E.Sauvage,
F.Jacquemotte,
K.Devriendt,
V.Tallon,
L.Ghosez,
and
J.M.Frère
(2005).
Specificity and reversibility of the transpeptidation reaction catalyzed by the Streptomyces R61 D-Ala-D-Ala peptidase.
|
| |
Protein Sci,
14,
2922-2928.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

