 |
PDBsum entry 1phh

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1phh
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.13.2
- 4-hydroxybenzoate 3-monooxygenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Benzoate Metabolism
|
 |
 |
 |
 |
 |
Reaction:
|
 |
4-hydroxybenzoate + NADPH + O2 + H+ = 3,4-dihydroxybenzoate + NADP+ + H2O
|
 |
 |
 |
 |
 |
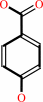 4-hydroxybenzoate
4-hydroxybenzoate
|
+
|
 NADPH
NADPH
|
+
|
 O2
O2
|
+
|
H(+)
|
=
|
3,4-dihydroxybenzoate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 NADP(+)
NADP(+)
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Mol Biol
199:637-648
(1988)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of p-hydroxybenzoate hydroxylase complexed with its reaction product 3,4-dihydroxybenzoate.
|
|
H.A.Schreuder,
J.M.van der Laan,
W.G.Hol,
J.Drenth.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Crystals of the flavin-containing enzyme p-hydroxybenzoate hydroxylase (PHBHase)
complexed with its reaction product were investigated in order to obtain insight
into the catalytic cycle of this enzyme involving two substrates and two
cofactors. PHBHase was crystallized initially with its substrate,
p-hydroxybenzoate and the substrate was then converted into the product
3,4-dihydroxybenzoate by allowing the catalytic reaction to proceed in the
crystals. In addition, crystals were soaked in mother liquor containing a high
concentration of this product. Data up to 2.3 A (1 A = 0.1 nm) were collected by
the oscillation method and the structure of the enzyme product complex was
refined by alternate restrained least-squares procedures and model building by
computer graphics techniques. A total of 273 solvent molecules could be located,
four of them being presumably sulfate ions. The R-factor for 14,339 reflections
between 6.0 A and 2.3 A is 19.3%. The 3-hydroxyl group of the product introduced
by the enzyme is clearly visible in the electron density, showing unambiguously
which carbon atom of the substrate is hydroxylated. A clear picture of the
hydroxylation site is obtained. The plane of the product is rotated 21 degrees
with respect to the plane of the substrate in the current model of
enzyme-substrate complex. The 4-hydroxyl group of the product is hydrogen bonded
to the hydroxyl group of Tyr201, its carboxyl group is interacting with the
side-chains of Tyr222, Arg214 and Ser212, while the newly introduced 3-hydroxyl
group makes a hydrogen bond with the backbone carbonyl oxygen of Pro293.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Y.W.Tan,
and
H.Yang
(2011).
Seeing the forest for the trees: fluorescence studies of single enzymes in the context of ensemble experiments.
|
| |
Phys Chem Chem Phys,
13,
1709-1721.
|
 |
|
|
|
|
 |
K.Ida,
M.Kurabayashi,
M.Suguro,
Y.Hiruma,
T.Hikima,
M.Yamomoto,
and
H.Suzuki
(2008).
Structural basis of proteolytic activation of L-phenylalanine oxidase from Pseudomonas sp. P-501.
|
| |
J Biol Chem,
283,
16584-16590.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
U.Theissen,
and
W.Martin
(2008).
Sulfide : quinone oxidoreductase (SQR) from the lugworm Arenicola marina shows cyanide- and thioredoxin-dependent activity.
|
| |
FEBS J,
275,
1131-1139.
|
 |
|
|
|
|
 |
A.Suemori,
and
M.Iwakura
(2007).
A systematic and comprehensive combinatorial approach to simultaneously improve the activity, reaction specificity, and thermal stability of p-hydroxybenzoate hydroxylase.
|
| |
J Biol Chem,
282,
19969-19978.
|
 |
|
|
|
|
 |
S.H.Kim,
T.Hisano,
K.Takeda,
W.Iwasaki,
A.Ebihara,
and
K.Miki
(2007).
Crystal structure of the oxygenase component (HpaB) of the 4-hydroxyphenylacetate 3-monooxygenase from Thermus thermophilus HB8.
|
| |
J Biol Chem,
282,
33107-33117.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Miyazaki,
K.Ohkubo,
T.Kojima,
and
S.Fukuzumi
(2007).
Modulation of characteristics of a ruthenium-coordinated flavin analogue that shows an unusual coordination mode.
|
| |
Angew Chem Int Ed Engl,
46,
905-908.
|
 |
|
|
|
|
 |
N.Gohain,
L.S.Thomashow,
D.V.Mavrodi,
and
W.Blankenfeldt
(2006).
The purification, crystallization and preliminary structural characterization of FAD-dependent monooxygenase PhzS, a phenazine-modifying enzyme from Pseudomonas aeruginosa.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
989-992.
|
 |
|
|
|
|
 |
B.A.Palfey,
R.Basu,
K.K.Frederick,
B.Entsch,
and
D.P.Ballou
(2002).
Role of protein flexibility in the catalytic cycle of p-hydroxybenzoate hydroxylase elucidated by the Pro293Ser mutant.
|
| |
Biochemistry,
41,
8438-8446.
|
 |
|
|
|
|
 |
R.M.Geha,
K.Chen,
J.Wouters,
F.Ooms,
and
J.C.Shih
(2002).
Analysis of conserved active site residues in monoamine oxidase A and B and their three-dimensional molecular modeling.
|
| |
J Biol Chem,
277,
17209-17216.
|
 |
|
|
|
|
 |
J.Beynon,
E.R.Rafanan,
B.Shen,
and
A.J.Fisher
(2000).
Crystallization and preliminary X-ray analysis of tetracenomycin A2 oxygenase: a flavoprotein hydroxylase involved in polyketide biosynthesis.
|
| |
Acta Crystallogr D Biol Crystallogr,
56,
1647-1651.
|
 |
|
|
|
|
 |
S.B.delCardayre,
and
J.E.Davies
(1998).
Staphylococcus aureus coenzyme A disulfide reductase, a new subfamily of pyridine nucleotide-disulfide oxidoreductase. Sequence, expression, and analysis of cdr.
|
| |
J Biol Chem,
273,
5752-5757.
|
 |
|
|
|
|
 |
F.J.van der Bolt,
R.H.van den Heuvel,
J.Vervoort,
and
W.J.van Berkel
(1997).
19F NMR study on the regiospecificity of hydroxylation of tetrafluoro-4-hydroxybenzoate by wild-type and Y385F p-hydroxybenzoate hydroxylase: evidence for a consecutive oxygenolytic dehalogenation mechanism.
|
| |
Biochemistry,
36,
14192-14201.
|
 |
|
|
|
|
 |
P.Chaiyen,
P.Brissette,
D.P.Ballou,
and
V.Massey
(1997).
Thermodynamics and reduction kinetics properties of 2-methyl-3-hydroxypyridine-5-carboxylic acid oxygenase.
|
| |
Biochemistry,
36,
2612-2621.
|
 |
|
|
|
|
 |
D.L.Gatti,
B.Entsch,
D.P.Ballou,
and
M.L.Ludwig
(1996).
pH-dependent structural changes in the active site of p-hydroxybenzoate hydroxylase point to the importance of proton and water movements during catalysis.
|
| |
Biochemistry,
35,
567-578.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.Pavone,
G.Gaeta,
A.Lombardi,
F.Nastri,
O.Maglio,
C.Isernia,
and
M.Saviano
(1996).
Discovering protein secondary structures: classification and description of isolated alpha-turns.
|
| |
Biopolymers,
38,
705-721.
|
 |
|
|
|
|
 |
H.R.Schläfli,
D.P.Baker,
T.Leisinger,
and
A.M.Cook
(1995).
Stereospecificity of hydride removal from NADH by reductases of multicomponent nonheme iron oxygenase systems.
|
| |
J Bacteriol,
177,
831-834.
|
 |
|
|
|
|
 |
M.Peräkylä,
and
T.A.Pakkanen
(1995).
Model assembly study of the ligand binding by p-hydroxybenzoate hydroxylase: correlation between the calculated binding energies and the experimental dissociation constants.
|
| |
Proteins,
21,
22-29.
|
 |
|
|
|
|
 |
M.T.Barakat,
and
P.M.Dean
(1995).
The atom assignment problem in automated de novo drug design. 3. Algorithms for optimization of fragment placement onto 3D molecular graphs.
|
| |
J Comput Aided Mol Des,
9,
359-372.
|
 |
|
|
|
|
 |
M.T.Barakat,
and
P.M.Dean
(1995).
The atom assignment problem in automated de novo drug design. 4. Tests for site-directed fragment placement based on molecular complementarity.
|
| |
J Comput Aided Mol Des,
9,
448-456.
|
 |
|
|
|
|
 |
T.Sandalova,
and
Y.Lindqvist
(1995).
Three-dimensional model of the alpha-subunit of bacterial luciferase.
|
| |
Proteins,
23,
241-255.
|
 |
|
|
|
|
 |
A.G.Schepky,
A.M.Schmidt,
T.Schmidt,
P.Schulz-Knappe,
and
W.G.Forssmann
(1994).
Determination of sulfated peptides by differential iodination.
|
| |
Biol Chem Hoppe Seyler,
375,
201-203.
|
 |
|
|
|
|
 |
M.J.Adams,
G.H.Ellis,
S.Gover,
C.E.Naylor,
and
C.Phillips
(1994).
Crystallographic study of coenzyme, coenzyme analogue and substrate binding in 6-phosphogluconate dehydrogenase: implications for NADP specificity and the enzyme mechanism.
|
| |
Structure,
2,
651-668.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.L.Chau,
and
P.M.Dean
(1994).
Electrostatic complementarity between proteins and ligands. 1. Charge disposition, dielectric and interface effects.
|
| |
J Comput Aided Mol Des,
8,
513-525.
|
 |
|
|
|
|
 |
P.L.Chau,
and
P.M.Dean
(1994).
Electrostatic complementarity between proteins and ligands. 2. Ligand moieties.
|
| |
J Comput Aided Mol Des,
8,
527-544.
|
 |
|
|
|
|
 |
P.L.Chau,
and
P.M.Dean
(1994).
Electrostatic complementarity between proteins and ligands. 3. Structural basis.
|
| |
J Comput Aided Mol Des,
8,
545-564.
|
 |
|
|
|
|
 |
M.E.Duban,
K.Lee,
and
D.G.Lynn
(1993).
Strategies in pathogenesis: mechanistic specificity in the detection of generic signals.
|
| |
Mol Microbiol,
7,
637-645.
|
 |
|
|
|
|
 |
H.A.Schreuder,
J.M.van der Laan,
M.B.Swarte,
K.H.Kalk,
W.G.Hol,
and
J.Drenth
(1992).
Crystal structure of the reduced form of p-hydroxybenzoate hydroxylase refined at 2.3 A resolution.
|
| |
Proteins,
14,
178-190.
|
 |
|
|
|
|
 |
W.van Berkel,
A.Westphal,
K.Eschrich,
M.Eppink,
and
A.de Kok
(1992).
Substitution of Arg214 at the substrate-binding site of p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens.
|
| |
Eur J Biochem,
210,
411-419.
|
 |
|
|
|
|
 |
J.Vervoort,
W.J.Van Berkel,
F.Müller,
and
C.T.Moonen
(1991).
NMR studies on p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens and salicylate hydroxylase from Pseudomonas putida.
|
| |
Eur J Biochem,
200,
731-738.
|
 |
|
|
|
|
 |
M.Suzuki,
T.Hayakawa,
J.P.Shaw,
M.Rekik,
and
S.Harayama
(1991).
Primary structure of xylene monooxygenase: similarities to and differences from the alkane hydroxylation system.
|
| |
J Bacteriol,
173,
1690-1695.
|
 |
|
|
|
|
 |
M.S.Johnson,
M.J.Sutcliffe,
and
T.L.Blundell
(1990).
Molecular anatomy: phyletic relationships derived from three-dimensional structures of proteins.
|
| |
J Mol Evol,
30,
43-59.
|
 |
|
|
|
|
 |
F.M.Vellieux,
F.Huitema,
H.Groendijk,
K.H.Kalk,
J.F.Jzn,
J.A.Jongejan,
J.A.Duine,
K.Petratos,
J.Drenth,
and
W.G.Hol
(1989).
Structure of quinoprotein methylamine dehydrogenase at 2.25 A resolution.
|
| |
EMBO J,
8,
2171-2178.
|
 |
|
|
|
|
 |
S.R.Presnell,
and
F.E.Cohen
(1989).
Topological distribution of four-alpha-helix bundles.
|
| |
Proc Natl Acad Sci U S A,
86,
6592-6596.
|
 |
|
|
|
|
 |
W.J.Van Berkel,
and
F.Müller
(1989).
The temperature and pH dependence of some properties of p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens.
|
| |
Eur J Biochem,
179,
307-314.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

