 |
PDBsum entry 1ojc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1ojc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.1.4.3.21
- primary-amine oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a primary methyl amine + O2 + H2O = an aldehyde + H2O2 + NH4+
|
 |
 |
 |
 |
 |
primary methyl amine
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
 aldehyde
aldehyde
|
+
|
 H2O2
H2O2
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.1.4.3.4
- monoamine oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a secondary aliphatic amine + O2 + H2O = a primary amine + an aldehyde + H2O2
|
 |
 |
 |
 |
 |
secondary aliphatic amine
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
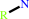 primary amine
primary amine
|
+
|
 aldehyde
aldehyde
|
+
|
 H2O2
H2O2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
|
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
100:9750-9755
(2003)
|
|
PubMed id:
|
|
|
|

|
| |
|
Insights into the mode of inhibition of human mitochondrial monoamine oxidase B from high-resolution crystal structures.
|
|
C.Binda,
M.Li,
F.Hubalek,
N.Restelli,
D.E.Edmondson,
A.Mattevi.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Monoamine oxidase B (MAO-B) is an outer mitochondrial membrane-bound enzyme that
catalyzes the oxidative deamination of arylalkylamine neurotransmitters and has
been a target for a number of clinically used drug inhibitors. The 1.7-A
structure of the reversible isatin-MAO-B complex has been determined; it forms a
basis for the interpretation of the enzyme's structure when bound to either
reversible or irreversible inhibitors. 1,4-Diphenyl-2-butene is found to be a
reversible MAO-B inhibitor, which occupies both the entrance and substrate
cavity space in the enzyme. Comparison of these two structures identifies
Ile-199 as a "gate" between the two cavities. Rotation of the side
chain allows for either separation or fusion of the two cavities. Inhibition of
the enzyme with N-(2-aminoethyl)-p-chlorobenzamide results in the formation of a
covalent N(5) flavin adduct with the phenyl ring of the inhibitor occupying a
position in the catalytic site overlapping that of isatin. Inhibition of MAO-B
with the clinically used trans-2-phenylcyclopropylamine results in the formation
of a covalent C(4a) flavin adduct with an opened cyclopropyl ring and the phenyl
ring in a parallel orientation to the flavin. The peptide bond between the
flavin-substituted Cys-397 and Tyr-398 is in a cis conformation, which allows
the proper orientation of the phenolic ring of Tyr-398 in the active site. The
flavin ring exists in a twisted nonplanar conformation, which is observed in the
oxidized form as well as in both the N(5) and the C(4a) adducts. An immobile
water molecule is H-bonded to Lys-296 and to the N(5) of the flavin as observed
in other flavin-dependent amine oxidases. The active site cavities are highly
apolar; however, hydrophilic areas exist near the flavin and direct the amine
moiety of the substrate for binding and catalysis. Small conformational changes
are observed on comparison of the different inhibitor-enzyme complexes. Future
MAO-B drug design will need to consider "induced fit" contributions as
an element in ligand-enzyme interactions.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Fig. 2. Structures of MAO-B inhibitors used in this study
and atomic numbering of the flavin ring.
|
 |
Figure 4.
Fig. 4. LIGPLOT (15) illustration of 1,4-diphenyl-2-butene
binding to MAO-B. Dashed lines indicate H-bonds. Carbons are in
black, nitrogens in blue, oxygens in red, and sulfurs in yellow.
Water molecules are shown as cyan spheres.
|
 |
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.Aldeco,
B.K.Arslan,
and
D.E.Edmondson
(2011).
Catalytic and inhibitor binding properties of zebrafish monoamine oxidase (zMAO): comparisons with human MAO A and MAO B.
|
| |
Comp Biochem Physiol B Biochem Mol Biol,
159,
78-83.
|
 |
|
|
|
|
 |
M.S.Jorns,
Z.W.Chen,
and
F.S.Mathews
(2010).
Structural characterization of mutations at the oxygen activation site in monomeric sarcosine oxidase .
|
| |
Biochemistry,
49,
3631-3639.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
O.Buneeva,
O.Gnedenko,
V.Zgoda,
A.Kopylov,
V.Glover,
A.Ivanov,
A.Medvedev,
and
A.Archakov
(2010).
Isatin-binding proteins of rat and mouse brain: proteomic identification and optical biosensor validation.
|
| |
Proteomics,
10,
23-37.
|
 |
|
|
|
|
 |
P.F.Fitzpatrick
(2010).
Oxidation of amines by flavoproteins.
|
| |
Arch Biochem Biophys,
493,
13-25.
|
 |
|
|
|
|
 |
A.Karytinos,
F.Forneris,
A.Profumo,
G.Ciossani,
E.Battaglioli,
C.Binda,
and
A.Mattevi
(2009).
A novel mammalian flavin-dependent histone demethylase.
|
| |
J Biol Chem,
284,
17775-17782.
|
 |
|
|
|
|
 |
D.E.Edmondson,
C.Binda,
J.Wang,
A.K.Upadhyay,
and
A.Mattevi
(2009).
Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases.
|
| |
Biochemistry,
48,
4220-4230.
|
 |
|
|
|
|
 |
E.M.Van der Walt,
E.M.Milczek,
S.F.Malan,
D.E.Edmondson,
N.Castagnoli,
J.J.Bergh,
and
J.P.Petzer
(2009).
Inhibition of monoamine oxidase by (E)-styrylisatin analogues.
|
| |
Bioorg Med Chem Lett,
19,
2509-2513.
|
 |
|
|
|
|
 |
M.Naoi,
and
W.Maruyama
(2009).
Functional mechanism of neuroprotection by inhibitors of type B monoamine oxidase in Parkinson's disease.
|
| |
Expert Rev Neurother,
9,
1233-1250.
|
 |
|
|
|
|
 |
D.B.Langley,
D.M.Trambaiolo,
A.P.Duff,
D.M.Dooley,
H.C.Freeman,
and
J.M.Guss
(2008).
Complexes of the copper-containing amine oxidase from Arthrobacter globiformis with the inhibitors benzylhydrazine and tranylcypromine.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
64,
577-583.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.M.Milczek,
D.Bonivento,
C.Binda,
A.Mattevi,
I.A.McDonald,
and
D.E.Edmondson
(2008).
Structural and mechanistic studies of mofegiline inhibition of recombinant human monoamine oxidase B.
|
| |
J Med Chem,
51,
8019-8026.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
F.Forneris,
C.Binda,
E.Battaglioli,
and
A.Mattevi
(2008).
LSD1: oxidative chemistry for multifaceted functions in chromatin regulation.
|
| |
Trends Biochem Sci,
33,
181-189.
|
 |
|
|
|
|
 |
G.Zhao,
R.C.Bruckner,
and
M.S.Jorns
(2008).
Identification of the oxygen activation site in monomeric sarcosine oxidase: role of Lys265 in catalysis.
|
| |
Biochemistry,
47,
9124-9135.
|
 |
|
|
|
|
 |
K.E.Atkin,
R.Reiss,
N.J.Turner,
A.M.Brzozowski,
and
G.Grogan
(2008).
Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of variants of monoamine oxidase from Aspergillus niger.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
64,
182-185.
|
 |
|
|
|
|
 |
M.O.Ogunrombi,
S.F.Malan,
G.Terre'blanche,
N.Castagnoli,
J.J.Bergh,
and
J.P.Petzer
(2008).
Structure-activity relationships in the inhibition of monoamine oxidase B by 1-methyl-3-phenylpyrroles.
|
| |
Bioorg Med Chem,
16,
2463-2472.
|
 |
|
|
|
|
 |
S.Hruschka,
T.C.Rosen,
S.Yoshida,
K.L.Kirk,
R.Fröhlich,
B.Wibbeling,
and
G.Haufe
(2008).
Fluorinated phenylcyclopropylamines. Part 5: Effects of electron-withdrawing or -donating aryl substituents on the inhibition of monoamine oxidases A and B by 2-aryl-2-fluoro-cyclopropylamines.
|
| |
Bioorg Med Chem,
16,
7148-7166.
|
 |
|
|
|
|
 |
S.Y.Son,
J.Ma,
Y.Kondou,
M.Yoshimura,
E.Yamashita,
and
T.Tsukihara
(2008).
Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/inhibitors.
|
| |
Proc Natl Acad Sci U S A,
105,
5739-5744.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Fierro,
M.Osorio-Olivares,
B.K.Cassels,
D.E.Edmondson,
S.Sepúlveda-Boza,
and
M.Reyes-Parada
(2007).
Human and rat monoamine oxidase-A are differentially inhibited by (S)-4-alkylthioamphetamine derivatives: insights from molecular modeling studies.
|
| |
Bioorg Med Chem,
15,
5198-5206.
|
 |
|
|
|
|
 |
A.Medvedev,
O.Buneeva,
and
V.Glover
(2007).
Biological targets for isatin and its analogues: Implications for therapy.
|
| |
Biologics,
1,
151-162.
|
 |
|
|
|
|
 |
D.E.Edmondson,
C.Binda,
and
A.Mattevi
(2007).
Structural insights into the mechanism of amine oxidation by monoamine oxidases A and B.
|
| |
Arch Biochem Biophys,
464,
269-276.
|
 |
|
|
|
|
 |
D.E.Edmondson,
L.DeColibus,
C.Binda,
M.Li,
and
A.Mattevi
(2007).
New insights into the structures and functions of human monoamine oxidases A and B.
|
| |
J Neural Transm,
114,
703-705.
|
 |
|
|
|
|
 |
E.C.Ralph,
J.S.Hirschi,
M.A.Anderson,
W.W.Cleland,
D.A.Singleton,
and
P.F.Fitzpatrick
(2007).
Insights into the mechanism of flavoprotein-catalyzed amine oxidation from nitrogen isotope effects on the reaction of N-methyltryptophan oxidase.
|
| |
Biochemistry,
46,
7655-7664.
|
 |
|
|
|
|
 |
J.C.Shih
(2007).
Monoamine oxidases: from tissue homogenates to transgenic mice.
|
| |
Neurochem Res,
32,
1757-1761.
|
 |
|
|
|
|
 |
M.A.Akyüz,
S.S.Erdem,
and
D.E.Edmondson
(2007).
The aromatic cage in the active site of monoamine oxidase B: effect on the structural and electronic properties of bound benzylamine and p-nitrobenzylamine.
|
| |
J Neural Transm,
114,
693-698.
|
 |
|
|
|
|
 |
A.A.Khalil,
B.Davies,
and
N.Castagnoli
(2006).
Isolation and characterization of a monoamine oxidase B selective inhibitor from tobacco smoke.
|
| |
Bioorg Med Chem,
14,
3392-3398.
|
 |
|
|
|
|
 |
A.Carotti,
C.Altomare,
M.Catto,
C.Gnerre,
L.Summo,
A.De Marco,
S.Rose,
P.Jenner,
and
B.Testa
(2006).
Lipophilicity plays a major role in modulating the inhibition of monoamine oxidase B by 7-substituted coumarins.
|
| |
Chem Biodivers,
3,
134-149.
|
 |
|
|
|
|
 |
A.Nagpal,
M.P.Valley,
P.F.Fitzpatrick,
and
A.M.Orville
(2006).
Crystal structures of nitroalkane oxidase: insights into the reaction mechanism from a covalent complex of the flavoenzyme trapped during turnover.
|
| |
Biochemistry,
45,
1138-1150.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.G.Lee,
C.Wynder,
D.A.Bochar,
M.A.Hakimi,
N.Cooch,
and
R.Shiekhattar
(2006).
Functional interplay between histone demethylase and deacetylase enzymes.
|
| |
Mol Cell Biol,
26,
6395-6402.
|
 |
|
|
|
|
 |
N.Vlok,
S.F.Malan,
N.Castagnoli,
J.J.Bergh,
and
J.P.Petzer
(2006).
Inhibition of monoamine oxidase B by analogues of the adenosine A2A receptor antagonist (E)-8-(3-chlorostyryl)caffeine (CSC).
|
| |
Bioorg Med Chem,
14,
3512-3521.
|
 |
|
|
|
|
 |
S.S.Erdem,
O.Karahan,
I.Yildiz,
and
K.Yelekçi
(2006).
A computational study on the amine-oxidation mechanism of monoamine oxidase: insight into the polar nucleophilic mechanism.
|
| |
Org Biomol Chem,
4,
646-658.
|
 |
|
|
|
|
 |
C.Binda,
F.Hubálek,
M.Li,
Y.Herzig,
J.Sterling,
D.E.Edmondson,
and
A.Mattevi
(2005).
Binding of rasagiline-related inhibitors to human monoamine oxidases: a kinetic and crystallographic analysis.
|
| |
J Med Chem,
48,
8148-8154.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.De Colibus,
M.Li,
C.Binda,
A.Lustig,
D.E.Edmondson,
and
A.Mattevi
(2005).
Three-dimensional structure of human monoamine oxidase A (MAO A): relation to the structures of rat MAO A and human MAO B.
|
| |
Proc Natl Acad Sci U S A,
102,
12684-12689.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.G.Lee,
C.Wynder,
N.Cooch,
and
R.Shiekhattar
(2005).
An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation.
|
| |
Nature,
437,
432-435.
|
 |
|
|
|
|
 |
J.Ma,
F.Kubota,
M.Yoshimura,
E.Yamashita,
A.Nakagawa,
A.Ito,
and
T.Tsukihara
(2004).
Crystallization and preliminary crystallographic analysis of rat monoamine oxidase A complexed with clorgyline.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
317-319.
|
 |
|
|
|
|
 |
K.Chen,
D.P.Holschneider,
W.Wu,
I.Rebrin,
and
J.C.Shih
(2004).
A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior.
|
| |
J Biol Chem,
279,
39645-39652.
|
 |
|
|
|
|
 |
T.C.Rosen,
S.Yoshida,
K.L.Kirk,
and
G.Haufe
(2004).
Fluorinated phenylcyclopropylamines as inhibitors of monoamine oxidases.
|
| |
Chembiochem,
5,
1033-1043.
|
 |
|
|
|
|
 |
Z.Yang,
L.Shipman,
M.Zhang,
B.P.Anton,
R.J.Roberts,
and
X.Cheng
(2004).
Structural characterization and comparative phylogenetic analysis of Escherichia coli HemK, a protein (N5)-glutamine methyltransferase.
|
| |
J Mol Biol,
340,
695-706.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

