 |
PDBsum entry 1ocv

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Ketosteroid isomerase
|
PDB id
|
|

|

|
1ocv
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.5.3.3.1
- steroid Delta-isomerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 3-oxo-Delta5-steroid = a 3-oxo-Delta4-steroid
|
 |
 |
 |
 |
 |
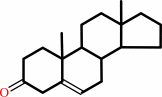 3-oxo-Delta(5)-steroid
3-oxo-Delta(5)-steroid
|
=
|
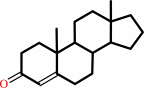 3-oxo-Delta(4)-steroid
3-oxo-Delta(4)-steroid
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
278:28229-28236
(2003)
|
|
PubMed id:
|
|
|
|

|
| |
|
Origin of the different pH activity profile in two homologous ketosteroid isomerases.
|
|
Y.S.Yun,
T.H.Lee,
G.H.Nam,
d.o. .S.Jang,
S.Shin,
B.H.Oh,
K.Y.Choi.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Two homologous Delta5-3-ketosteroid isomerases from Comamonas testosteroni
(TI-WT) and Pseudomonas putida biotype B (PI-WT) exhibit different pH activity
profiles. TI-WT loses activity below pH 5.0 due to the protonation of the
conserved catalytic base, Asp-38, while PI-WT does not. Based on the structural
analysis of PI-WT, the critical catalytic base, Asp-38, was found to form a
hydrogen bond with the indole ring NH of Trp-116, which is homologously replaced
with Phe-116 in TI-WT. To investigate the role of Trp-116, we prepared the F116W
mutant of TI-WT (TI-F116W) and the W116F mutant of PI-WT (PI-W116F) and compared
kinetic parameters of those mutants at different pH levels. PI-W116F exhibited
significantly decreased catalytic activity at acidic pH like TI-WT, whereas
TI-F116W maintained catalytic activity at acidic pH like PI-WT and increased the
kcat/Km value by 2.5- to 4.7-fold compared with TI-WT at pH 3.8. The crystal
structure of TI-F116W clearly showed that the indole ring NH of Trp-116 could
form a hydrogen bond with the carboxyl oxygen of Asp-38 like that of PI-WT. The
present results demonstrate that the activities of both PI-WT and TI-F116W at
low pH were maintained by a tryptophan, which was able not only to lower the pKa
value of the catalytic base but also to increase the substrate affinity. This is
one example of the strategy nature can adopt to evolve the diversity of the
catalytic function in the enzymes. Our results provide insight into deciphering
the molecular evolution of the enzyme and creating novel enzymes by protein
engineering.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
FIG. 2. pH dependence of kinetic parameters of WT and
mutant KSIs. The lines represent nonlinear least-squares fits of
the data to Equations 1 and 2 to obtain the pK[E] and pK[ES]
values, respectively, as listed in Table I. 5,10-EST was used as
a substrate for graphs C and D, and for PI-WT in graphs A and B.
5-AND was used as a substrate for graphs E and F, and for
PI-W116F in graphs A and B.
|
 |
Figure 4.
FIG. 4. Stereoview of the catalytic base of TI-F116W with
2F[o]-F[c]-simulated annealing omit electron density map
contoured at 1.0  . Residues Trp-116 and
Asp-38, which were omitted from the model, display clear
electron density. A hydrogen bond between Asp-38 and Trp-116 is
represented by a dashed line. The figure was drawn by using the
program BobScript and rendered using Raster3D. . Residues Trp-116 and
Asp-38, which were omitted from the model, display clear
electron density. A hydrogen bond between Asp-38 and Trp-116 is
represented by a dashed line. The figure was drawn by using the
program BobScript and rendered using Raster3D.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2003,
278,
28229-28236)
copyright 2003.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Y.S.Yun,
G.H.Nam,
Y.G.Kim,
B.H.Oh,
and
K.Y.Choi
(2005).
Small exterior hydrophobic cluster contributes to conformational stability and steroid binding in ketosteroid isomerase from Pseudomonas putida biotype B.
|
| |
FEBS J,
272,
1999-2011.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.E.Iismaa,
S.Holman,
M.A.Wouters,
L.Lorand,
R.M.Graham,
and
A.Husain
(2003).
Evolutionary specialization of a tryptophan indole group for transition-state stabilization by eukaryotic transglutaminases.
|
| |
Proc Natl Acad Sci U S A,
100,
12636-12641.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

