 |
PDBsum entry 1nx9

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.43
- alpha-amino-acid esterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
an alpha-amino acid ester + H2O = an alpha-amino acid + an alcohol + H+
|
 |
 |
 |
 |
 |
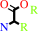 alpha-amino acid ester
alpha-amino acid ester
|
+
|
H2O
|
=
|
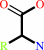 alpha-amino acid
alpha-amino acid
|
+
|
 alcohol
alcohol
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
281:5804-5810
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Acetobacter turbidans alpha-amino acid ester hydrolase: how a single mutation improves an antibiotic-producing enzyme.
|
|
T.R.Barends,
J.J.Polderman-Tijmes,
P.A.Jekel,
C.Williams,
G.Wybenga,
D.B.Janssen,
B.W.Dijkstra.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The alpha-amino acid ester hydrolase (AEH) from Acetobacter turbidans is a
bacterial enzyme catalyzing the hydrolysis and synthesis of beta-lactam
antibiotics. The crystal structures of the native enzyme, both unliganded and in
complex with the hydrolysis product D-phenylglycine are reported, as well as the
structures of an inactive mutant (S205A) complexed with the substrate
ampicillin, and an active site mutant (Y206A) with an increased tendency to
catalyze antibiotic production rather than hydrolysis. The structure of the
native enzyme shows an acyl binding pocket, in which D-phenylglycine binds, and
an additional space that is large enough to accommodate the beta-lactam moiety
of an antibiotic. In the S205A mutant, ampicillin binds in this pocket in a
non-productive manner, making extensive contacts with the side chain of
Tyr(112), which also participates in oxyanion hole formation. In the Y206A
mutant, the Tyr(112) side chain has moved with its hydroxyl group toward the
catalytic serine. Because this changes the properties of the beta-lactam binding
site, this could explain the increased beta-lactam transferase activity of this
mutant.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
a, stereo figure of the A. turbidans α-amino acid ester
hydrolase tetramer. A surface representation is shown, in which
each monomer is individually colored. b, stereo figure of the A.
turbidans α-amino acid ester hydrolase monomer. The arm and
α/β-hydrolase domains are shown in green, the cap domain in
orange, and the jellyroll domain in blue. The side chain of the
active site Ser^205 is shown in space filling representation.
Helices αC-αF and strands β4-β7 are labeled. The figure was
prepared using PYMOL (DeLano Scientific) and Molscript (30).
|
 |
Figure 4.
Stereo picture of the 2F[o] - F[c] electron density of
ampicillin in the active site of the S205A mutant, after
refinement of the initial molecular replacement solution, prior
to inclusion of ampicillin in the model. The density was
contoured at 1.0 σ, and overlaid on the final refined
structure. The figure was prepared using Xfit (22) and Raster3D
(32).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2006,
281,
5804-5810)
copyright 2006.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.Narasimhan,
M.R.Nance,
D.Gao,
M.C.Ko,
J.Macdonald,
P.Tamburi,
D.Yoon,
D.M.Landry,
J.H.Woods,
C.G.Zhan,
J.J.Tesmer,
and
R.K.Sunahara
(2010).
Structural analysis of thermostabilizing mutations of cocaine esterase.
|
| |
Protein Eng Des Sel,
23,
537-547.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

