|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
Chains A, B, C, D:
E.C.2.7.7.48
- RNA-directed Rna polymerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + a ribonucleoside 5'-triphosphate = RNA(n+1) + diphosphate
|
 |
 |
 |
 |
 |
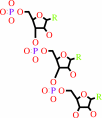 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
RNA(n+1)
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B, C, D:
E.C.3.4.21.98
- hepacivirin.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Hydrolysis of four peptide bonds in the viral precursor polyprotein, commonly with Asp or Glu in the P6 position, Cys or Thr in P1 and Ser or Ala in P1'.
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chains A, B, C, D:
E.C.3.4.22.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
Chains A, B, C, D:
E.C.3.6.1.15
- nucleoside-triphosphate phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a ribonucleoside 5'-triphosphate + H2O = a ribonucleoside 5'-diphosphate + phosphate + H+
|
 |
 |
 |
 |
 |
ribonucleoside 5'-triphosphate
|
+
|
H2O
|
=
|
ribonucleoside 5'-diphosphate
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 5:
|
 |
Chains A, B, C, D:
E.C.3.6.4.13
- Rna helicase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + H2O = ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
H2O
|
=
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Protein Sci
7:837-847
(1998)
|
|
PubMed id:
|
|
|
|

|
| |
|
Complex of NS3 protease and NS4A peptide of BK strain hepatitis C virus: a 2.2 A resolution structure in a hexagonal crystal form.
|
|
Y.Yan,
Y.Li,
S.Munshi,
V.Sardana,
J.L.Cole,
M.Sardana,
C.Steinkuehler,
L.Tomei,
R.De Francesco,
L.C.Kuo,
Z.Chen.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of the NS3 protease of the hepatitis C virus (BK strain)
has been determined in the space group P6(3)22 to a resolution of 2.2 A. This
protease is bound with a 14-mer peptide representing the central region of the
NS4A protein. There are two molecules of the NS3(1-180)-NS4A(21'-34') complex
per asymmetric unit. Each displays a familiar chymotrypsin-like fold that
includes two beta-barrel domains and four short alpha-helices. The catalytic
triad (Ser-139, His-57, and Asp-81) is located in the crevice between the
beta-barrel domains. The NS4A peptide forms an almost completely enclosed
peptide surface association with the protease. In contrast to the reported H
strain complex of NS3 protease-NS4A peptide in a trigonal crystal form (Kim JL
et al., 1996, Cell 87:343-355), the N-terminus of the NS3 protease is
well-ordered in both molecules in the asymmetric unit of our hexagonal crystal
form. The folding of the N-terminal region of the NS3 protease is due to the
formation of a three-helix bundle as a result of crystal packing. When compared
with the unbound structure (Love RA et al., 1996, Cell 87:331-342), the binding
of the NS4A peptide leads to the ordering of the N-terminal 28 residues of the
NS3 protease into a beta-strand and an alpha-helix and also causes local
rearrangements important for a catalytically favorable conformation at the
active site. Our analysis provides experimental support for the proposal that
binding of an NS4A-mimicking peptide, which increases catalytic rates, is
necessary but not sufficient for formation of a well-ordered, compact and,
hence, highly active protease molecule.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Peres-da-Silva,
A.J.de Almeida,
and
E.Lampe
(2010).
Mutations in hepatitis C virus NS3 protease domain associated with resistance to specific protease inhibitors in antiviral therapy naïve patients.
|
| |
Arch Virol,
155,
807-811.
|
 |
|
|
|
|
 |
A.J.Thompson,
and
J.G.McHutchison
(2009).
Review article: investigational agents for chronic hepatitis C.
|
| |
Aliment Pharmacol Ther,
29,
689-705.
|
 |
|
|
|
|
 |
A.Garcia-Pino,
M.Christensen-Dalsgaard,
L.Wyns,
M.Yarmolinsky,
R.D.Magnuson,
K.Gerdes,
and
R.Loris
(2008).
Doc of prophage P1 is inhibited by its antitoxin partner Phd through fold complementation.
|
| |
J Biol Chem,
283,
30821-30827.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Welsch,
F.S.Domingues,
S.Susser,
I.Antes,
C.Hartmann,
G.Mayr,
A.Schlicker,
C.Sarrazin,
M.Albrecht,
S.Zeuzem,
and
T.Lengauer
(2008).
Molecular basis of telaprevir resistance due to V36 and T54 mutations in the NS3-4A protease of the hepatitis C virus.
|
| |
Genome Biol,
9,
R16.
|
 |
|
|
|
|
 |
D.Luo,
T.Xu,
C.Hunke,
G.Grüber,
S.G.Vasudevan,
and
J.Lescar
(2008).
Crystal structure of the NS3 protease-helicase from dengue virus.
|
| |
J Virol,
82,
173-183.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.Brass,
J.M.Berke,
R.Montserret,
H.E.Blum,
F.Penin,
and
D.Moradpour
(2008).
Structural determinants for membrane association and dynamic organization of the hepatitis C virus NS3-4A complex.
|
| |
Proc Natl Acad Sci U S A,
105,
14545-14550.
|
 |
|
|
|
|
 |
W.Yang,
Y.Zhao,
J.Fabrycki,
X.Hou,
X.Nie,
A.Sanchez,
A.Phadke,
M.Deshpande,
A.Agarwal,
and
M.Huang
(2008).
Selection of replicon variants resistant to ACH-806, a novel hepatitis C virus inhibitor with no cross-resistance to NS3 protease and NS5B polymerase inhibitors.
|
| |
Antimicrob Agents Chemother,
52,
2043-2052.
|
 |
|
|
|
|
 |
A.E.Aleshin,
S.A.Shiryaev,
A.Y.Strongin,
and
R.C.Liddington
(2007).
Structural evidence for regulation and specificity of flaviviral proteases and evolution of the Flaviviridae fold.
|
| |
Protein Sci,
16,
795-806.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.D.Lindenbach,
B.M.Prágai,
R.Montserret,
R.K.Beran,
A.M.Pyle,
F.Penin,
and
C.M.Rice
(2007).
The C terminus of hepatitis C virus NS4A encodes an electrostatic switch that regulates NS5A hyperphosphorylation and viral replication.
|
| |
J Virol,
81,
8905-8918.
|
 |
|
|
|
|
 |
S.Franco,
M.Parera,
E.Aparicio,
B.Clotet,
and
M.A.Martinez
(2007).
Genetic and catalytic efficiency structure of an HCV protease quasispecies.
|
| |
Hepatology,
45,
899-910.
|
 |
|
|
|
|
 |
T.L.Tellinghuisen,
M.J.Evans,
T.von Hahn,
S.You,
and
C.M.Rice
(2007).
Studying hepatitis C virus: making the best of a bad virus.
|
| |
J Virol,
81,
8853-8867.
|
 |
|
|
|
|
 |
P.Toniutto,
C.Fabris,
and
M.Pirisi
(2006).
Antiviral treatment of hepatitis C.
|
| |
Expert Opin Pharmacother,
7,
2025-2035.
|
 |
|
|
|
|
 |
U.Kulkarni-Kale,
S.G.Bhosle,
G.S.Manjari,
M.Joshi,
S.Bansode,
and
A.S.Kolaskar
(2006).
Curation of viral genomes: challenges, applications and the way forward.
|
| |
BMC Bioinformatics,
7,
S12.
|
 |
|
|
|
|
 |
Y.Ding,
and
D.Wilkins
(2006).
Improving the Performance of SVM-RFE to Select Genes in Microarray Data.
|
| |
BMC Bioinformatics,
7,
S12.
|
 |
|
|
|
|
 |
C.Zhang,
Z.Cai,
Y.C.Kim,
R.Kumar,
F.Yuan,
P.Y.Shi,
C.Kao,
and
G.Luo
(2005).
Stimulation of hepatitis C virus (HCV) nonstructural protein 3 (NS3) helicase activity by the NS3 protease domain and by HCV RNA-dependent RNA polymerase.
|
| |
J Virol,
79,
8687-8697.
|
 |
|
|
|
|
 |
R.De Francesco,
and
G.Migliaccio
(2005).
Challenges and successes in developing new therapies for hepatitis C.
|
| |
Nature,
436,
953-960.
|
 |
|
|
|
|
 |
S.Vallet,
S.Gouriou,
J.B.Nousbaum,
M.C.Legrand-Quillien,
A.Goudeau,
and
B.Picard
(2005).
Genetic heterogeneity of the NS3 protease gene in hepatitis C virus genotype 1 from untreated infected patients.
|
| |
J Med Virol,
75,
528-537.
|
 |
|
|
|
|
 |
D.Thibeault,
C.Bousquet,
R.Gingras,
L.Lagacé,
R.Maurice,
P.W.White,
and
D.Lamarre
(2004).
Sensitivity of NS3 serine proteases from hepatitis C virus genotypes 2 and 3 to the inhibitor BILN 2061.
|
| |
J Virol,
78,
7352-7359.
|
 |
|
|
|
|
 |
F.Penin,
J.Dubuisson,
F.A.Rey,
D.Moradpour,
and
J.M.Pawlotsky
(2004).
Structural biology of hepatitis C virus.
|
| |
Hepatology,
39,
5.
|
 |
|
|
|
|
 |
L.Lu,
T.J.Pilot-Matias,
K.D.Stewart,
J.T.Randolph,
R.Pithawalla,
W.He,
P.P.Huang,
L.L.Klein,
H.Mo,
and
A.Molla
(2004).
Mutations conferring resistance to a potent hepatitis C virus serine protease inhibitor in vitro.
|
| |
Antimicrob Agents Chemother,
48,
2260-2266.
|
 |
|
|
|
|
 |
P.Niyomrattanakit,
P.Winoyanuwattikun,
S.Chanprapaph,
C.Angsuthanasombat,
S.Panyim,
and
G.Katzenmeier
(2004).
Identification of residues in the dengue virus type 2 NS2B cofactor that are critical for NS3 protease activation.
|
| |
J Virol,
78,
13708-13716.
|
 |
|
|
|
|
 |
W.Wang,
F.C.Lahser,
M.Yi,
J.Wright-Minogue,
E.Xia,
P.C.Weber,
S.M.Lemon,
and
B.A.Malcolm
(2004).
Conserved C-terminal threonine of hepatitis C virus NS3 regulates autoproteolysis and prevents product inhibition.
|
| |
J Virol,
78,
700-709.
|
 |
|
|
|
|
 |
Y.S.Tsantrizos
(2004).
The design of a potent inhibitor of the hepatitis C virus NS3 protease: BILN 2061--from the NMR tube to the clinic.
|
| |
Biopolymers,
76,
309-323.
|
 |
|
|
|
|
 |
C.Trozzi,
L.Bartholomew,
A.Ceccacci,
G.Biasiol,
L.Pacini,
S.Altamura,
F.Narjes,
E.Muraglia,
G.Paonessa,
U.Koch,
R.De Francesco,
C.Steinkuhler,
and
G.Migliaccio
(2003).
In vitro selection and characterization of hepatitis C virus serine protease variants resistant to an active-site peptide inhibitor.
|
| |
J Virol,
77,
3669-3679.
|
 |
|
|
|
|
 |
F.Narjes,
U.Koch,
and
C.Steinkühler
(2003).
Recent developments in the discovery of hepatitis C virus serine protease inhibitors--towards a new class of antiviral agents?
|
| |
Expert Opin Investig Drugs,
12,
153-163.
|
 |
|
|
|
|
 |
M.Dimitrova,
I.Imbert,
M.P.Kieny,
and
C.Schuster
(2003).
Protein-protein interactions between hepatitis C virus nonstructural proteins.
|
| |
J Virol,
77,
5401-5414.
|
 |
|
|
|
|
 |
M.Lundin,
M.Monné,
A.Widell,
G.Von Heijne,
and
M.A.Persson
(2003).
Topology of the membrane-associated hepatitis C virus protein NS4B.
|
| |
J Virol,
77,
5428-5438.
|
 |
|
|
|
|
 |
M.P.Walker,
N.Yao,
and
Z.Hong
(2003).
Promising candidates for the treatment of chronic hepatitis C.
|
| |
Expert Opin Investig Drugs,
12,
1269-1280.
|
 |
|
|
|
|
 |
A.Casbarra,
F.D.Piaz,
P.Ingallinella,
S.Orrù,
P.Pucci,
A.Pessi,
and
E.Bianchi
(2002).
The effect of prime-site occupancy on the hepatitis C virus NS3 protease structure.
|
| |
Protein Sci,
11,
2102-2112.
|
 |
|
|
|
|
 |
E.Bianchi,
and
A.Pessi
(2002).
Inhibiting viral proteases: challenges and opportunities.
|
| |
Biopolymers,
66,
101-114.
|
 |
|
|
|
|
 |
P.Ingallinella,
D.Fattori,
S.Altamura,
C.Steinkühler,
U.Koch,
D.Cicero,
R.Bazzo,
R.Cortese,
E.Bianchi,
and
A.Pessi
(2002).
Prime site binding inhibitors of a serine protease: NS3/4A of hepatitis C virus.
|
| |
Biochemistry,
41,
5483-5492.
|
 |
|
|
|
|
 |
A.Pessi
(2001).
A personal account of the role of peptide research in drug discovery: the case of hepatitis C.
|
| |
J Pept Sci,
7,
2.
|
 |
|
|
|
|
 |
B.W.Dymock
(2001).
Emerging therapies for hepatitis C virus infection.
|
| |
Expert Opin Emerg Drugs,
6,
13-42.
|
 |
|
|
|
|
 |
T.Heintges,
J.Encke,
J.zu Putlitz,
and
J.R.Wands
(2001).
Inhibition of hepatitis C virus NS3 function by antisense oligodeoxynucleotides and protease inhibitor.
|
| |
J Med Virol,
65,
671-680.
|
 |
|
|
|
|
 |
U.Koch,
G.Biasiol,
M.Brunetti,
D.Fattori,
M.Pallaoro,
and
C.Steinkühler
(2001).
Role of charged residues in the catalytic mechanism of hepatitis C virus NS3 protease: electrostatic precollision guidance and transition-state stabilization.
|
| |
Biochemistry,
40,
631-640.
|
 |
|
|
|
|
 |
B.Wölk,
D.Sansonno,
H.G.Kräusslich,
F.Dammacco,
C.M.Rice,
H.E.Blum,
and
D.Moradpour
(2000).
Subcellular localization, stability, and trans-cleavage competence of the hepatitis C virus NS3-NS4A complex expressed in tetracycline-regulated cell lines.
|
| |
J Virol,
74,
2293-2304.
|
 |
|
|
|
|
 |
F.Narjes,
M.Brunetti,
S.Colarusso,
B.Gerlach,
U.Koch,
G.Biasiol,
D.Fattori,
R.De Francesco,
V.G.Matassa,
and
C.Steinkühler
(2000).
Alpha-ketoacids are potent slow binding inhibitors of the hepatitis C virus NS3 protease.
|
| |
Biochemistry,
39,
1849-1861.
|
 |
|
|
|
|
 |
G.Barbato,
D.O.Cicero,
F.Cordier,
F.Narjes,
B.Gerlach,
S.Sambucini,
S.Grzesiek,
V.G.Matassa,
R.De Francesco,
and
R.Bazzo
(2000).
Inhibitor binding induces active site stabilization of the HCV NS3 protein serine protease domain.
|
| |
EMBO J,
19,
1195-1206.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
N.Butkiewicz,
N.Yao,
W.Zhong,
J.Wright-Minogue,
P.Ingravallo,
R.Zhang,
J.Durkin,
D.N.Standring,
B.M.Baroudy,
D.V.Sangar,
S.M.Lemon,
J.Y.Lau,
and
Z.Hong
(2000).
Virus-specific cofactor requirement and chimeric hepatitis C virus/GB virus B nonstructural protein 3.
|
| |
J Virol,
74,
4291-4301.
|
 |
|
|
|
|
 |
P.Ingallinella,
E.Bianchi,
R.Ingenito,
U.Koch,
C.Steinkühler,
S.Altamura,
and
A.Pessi
(2000).
Optimization of the P'-region of peptide inhibitors of hepatitis C virus NS3/4A protease.
|
| |
Biochemistry,
39,
12898-12906.
|
 |
|
|
|
|
 |
P.Leyssen,
E.De Clercq,
and
J.Neyts
(2000).
Perspectives for the treatment of infections with Flaviviridae.
|
| |
Clin Microbiol Rev,
13,
67.
|
 |
|
|
|
|
 |
T.Ueno,
S.Misawa,
Y.Ohba,
M.Matsumoto,
M.Mizunuma,
N.Kasai,
K.Tsumoto,
I.Kumagai,
and
H.Hayashi
(2000).
Isolation and characterization of monoclonal antibodies that inhibit hepatitis C virus NS3 protease.
|
| |
J Virol,
74,
6300-6308.
|
 |
|
|
|
|
 |
V.C.Lai,
W.Zhong,
A.Skelton,
P.Ingravallo,
V.Vassilev,
R.O.Donis,
Z.Hong,
and
J.Y.Lau
(2000).
Generation and characterization of a hepatitis C virus NS3 protease-dependent bovine viral diarrhea virus.
|
| |
J Virol,
74,
6339-6347.
|
 |
|
|
|
|
 |
A.Urbani,
G.Biasiol,
M.Brunetti,
C.Volpari,
S.Di Marco,
M.Sollazzo,
S.Orrú,
F.D.Piaz,
A.Casbarra,
P.Pucci,
C.Nardi,
P.Gallinari,
R.De Francesco,
and
C.Steinkühler
(1999).
Multiple determinants influence complex formation of the hepatitis C virus NS3 protease domain with its NS4A cofactor peptide.
|
| |
Biochemistry,
38,
5206-5215.
|
 |
|
|
|
|
 |
A.Y.Howe,
R.Chase,
S.S.Taremi,
C.Risano,
B.Beyer,
B.Malcolm,
and
J.Y.Lau
(1999).
A novel recombinant single-chain hepatitis C virus NS3-NS4A protein with improved helicase activity.
|
| |
Protein Sci,
8,
1332-1341.
|
 |
|
|
|
|
 |
E.Ferrari,
J.Wright-Minogue,
J.W.Fang,
B.M.Baroudy,
J.Y.Lau,
and
Z.Hong
(1999).
Characterization of soluble hepatitis C virus RNA-dependent RNA polymerase expressed in Escherichia coli.
|
| |
J Virol,
73,
1649-1654.
|
 |
|
|
|
|
 |
G.Filocamo,
L.Pacini,
C.Nardi,
L.Bartholomew,
M.Scaturro,
P.Delmastro,
A.Tramontano,
R.De Francesco,
and
G.Migliaccio
(1999).
Selection of functional variants of the NS3-NS4A protease of hepatitis C virus by using chimeric sindbis viruses.
|
| |
J Virol,
73,
561-575.
|
 |
|
|
|
|
 |
J.O.Koch,
and
R.Bartenschlager
(1999).
Modulation of hepatitis C virus NS5A hyperphosphorylation by nonstructural proteins NS3, NS4A, and NS4B.
|
| |
J Virol,
73,
7138-7146.
|
 |
|
|
|
|
 |
P.Gallinari,
C.Paolini,
D.Brennan,
C.Nardi,
C.Steinkühler,
and
R.De Francesco
(1999).
Modulation of hepatitis C virus NS3 protease and helicase activities through the interaction with NS4A.
|
| |
Biochemistry,
38,
5620-5632.
|
 |
|
|
|
|
 |
S.Orrù,
F.Dal Piaz,
A.Casbarra,
G.Biasiol,
R.De Francesco,
C.Steinkühler,
and
P.Pucci
(1999).
Conformational changes in the NS3 protease from hepatitis C virus strain Bk monitored by limited proteolysis and mass spectrometry.
|
| |
Protein Sci,
8,
1445-1454.
|
 |
|
|
|
|
 |
Y.Kusov,
and
V.Gauss-Müller
(1999).
Improving proteolytic cleavage at the 3A/3B site of the hepatitis A virus polyprotein impairs processing and particle formation, and the impairment can be complemented in trans by 3AB and 3ABC.
|
| |
J Virol,
73,
9867-9878.
|
 |
|
|
|
|
 |
C.Steinkühler,
G.Biasiol,
M.Brunetti,
A.Urbani,
U.Koch,
R.Cortese,
A.Pessi,
and
R.De Francesco
(1998).
Product inhibition of the hepatitis C virus NS3 protease.
|
| |
Biochemistry,
37,
8899-8905.
|
 |
|
|
|
|
 |
S.S.Taremi,
B.Beyer,
M.Maher,
N.Yao,
W.Prosise,
P.C.Weber,
and
B.A.Malcolm
(1998).
Construction, expression, and characterization of a novel fully activated recombinant single-chain hepatitis C virus protease.
|
| |
Protein Sci,
7,
2143-2149.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

