 |
PDBsum entry 1mzf

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1mzf
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.1.14.11.30
- hypoxia-inducible factor-asparagine dioxygenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-asparaginyl-[hypoxia-inducible factor alpha subunit] + 2-oxoglutarate + O2 = (3S)-3-hydroxy-L-asparaginyl-[hypoxia-inducible factor alpha subunit] + succinate + CO2
|
 |
 |
 |
 |
 |
L-asparaginyl-[hypoxia-inducible factor alpha subunit]
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 2-oxoglutarate
2-oxoglutarate
|
+
|
 O2
O2
|
=
|
(3S)-3-hydroxy-L-asparaginyl-[hypoxia-inducible factor alpha subunit]
|
+
|
 succinate
succinate
|
+
|
 CO2
CO2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe(2+); L-ascorbate
|
 |
 |
 |
 |
 |
Fe(2+)
|
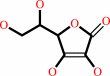 L-ascorbate
L-ascorbate
|
|
 |
 |
Enzyme class 2:
|
 |
E.C.1.14.11.n4
- ?????
|
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
99:15351-15356
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of factor-inhibiting hypoxia-inducible factor 1: An asparaginyl hydroxylase involved in the hypoxic response pathway.
|
|
C.E.Dann,
R.K.Bruick,
J.Deisenhofer.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Precise regulation of the evolutionarily conserved hypoxia-inducible
transcription factor (HIF) ensures proper adaptation to variations in oxygen
availability throughout development and into adulthood. Oxygen-dependent
regulation of HIF stability and activity are mediated by hydroxylation of
conserved proline and asparagine residues, respectively. Because the relevant
prolyl and asparginyl hydroxylases use O(2) to effect these posttranslational
modifications, these enzymes are implicated as direct oxygen sensors in the
mammalian hypoxic response pathway. Here we present the structure of
factor-inhibiting HIF-1 (FIH-1), the pertinent asparaginyl hydroxylase involved
in hypoxic signaling. Hydroxylation of the C-terminal transactivation domain
(CTAD) of HIF by FIH-1 prevents CTAD association with transcriptional
coactivators under normoxic conditions. Consistent with other structurally known
hydroxylases, FIH-1 is comprised of a beta-strand jellyroll core with both
Fe(II) and the cosubstrate 2-oxoglutarate bound in the active site. Details of
the molecular contacts at the active site of FIH-1 have been elucidated and
provide a platform for future drug design. Furthermore, the structure reveals
the presence of a FIH-1 homodimer that forms in solution and is essential for
FIH activity.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Fig 2. The primary structure of FIH-1 is labeled with
secondary structure elements taken from the x-ray
crystallographic model.  -strands and helices are
depicted as red arrows and yellow boxes, respectively. Residues
responsible for Fe(II) binding are highlighted in red, whereas
residues in close contact with 2-OG are highlighted in green. -strands and helices are
depicted as red arrows and yellow boxes, respectively. Residues
responsible for Fe(II) binding are highlighted in red, whereas
residues in close contact with 2-OG are highlighted in green.
|
 |
Figure 3.
Fig 3. FIH-1 structure contains a  -jellyroll core marked
by an extension of one of the -jellyroll core marked
by an extension of one of the  -sheets away from the
core and helices dotting the periphery. (A) A ribbon model of
the FIH-1 monomer is positioned looking between the -sheets away from the
core and helices dotting the periphery. (A) A ribbon model of
the FIH-1 monomer is positioned looking between the  -sheets
comprising the jellyroll and into the active site cavity. The
active site metal is shown as a red sphere. Structural elements
are colored as in Fig. 2. (B) A secondary structure topology
diagram shows the arrangement of the 14 -sheets
comprising the jellyroll and into the active site cavity. The
active site metal is shown as a red sphere. Structural elements
are colored as in Fig. 2. (B) A secondary structure topology
diagram shows the arrangement of the 14  -strands (triangles) and
8 helices (circles) in FIH-1. The core jellyroll motif,
structurally homologous to the cupin protein family, is colored
in red. (C) FIH-1 exists as a functionally relevant dimer in the
crystal. The first monomer of the dimer is colored as in A,
whereas the second monomer is blue. N and C termini are marked
as black circles. The figure was generated by using RIBBONS (41). -strands (triangles) and
8 helices (circles) in FIH-1. The core jellyroll motif,
structurally homologous to the cupin protein family, is colored
in red. (C) FIH-1 exists as a functionally relevant dimer in the
crystal. The first monomer of the dimer is colored as in A,
whereas the second monomer is blue. N and C termini are marked
as black circles. The figure was generated by using RIBBONS (41).
|
 |
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.Saban,
S.C.Flagg,
and
M.J.Knapp
(2011).
Uncoupled O2-activation in the human HIF-asparaginyl hydroxylase, FIH, does not produce reactive oxygen species.
|
| |
J Inorg Biochem,
105,
630-636.
|
 |
|
|
|
|
 |
M.Kato,
Y.Araiso,
A.Noma,
A.Nagao,
T.Suzuki,
R.Ishitani,
and
O.Nureki
(2011).
Crystal structure of a novel JmjC-domain-containing protein, TYW5, involved in tRNA modification.
|
| |
Nucleic Acids Res,
39,
1576-1585.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Moon,
S.Han,
H.Park,
and
J.Choe
(2010).
Crystal structures of human FIH-1 in complex with quinol family inhibitors.
|
| |
Mol Cells,
29,
471-474.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.S.Kim,
H.L.Kim,
K.H.Kim,
d.o. .J.Kim,
S.J.Lee,
J.Y.Yoon,
H.J.Yoon,
H.Y.Lee,
S.B.Park,
S.J.Kim,
J.Y.Lee,
and
S.W.Suh
(2010).
Crystal structure of Tpa1 from Saccharomyces cerevisiae, a component of the messenger ribonucleoprotein complex.
|
| |
Nucleic Acids Res,
38,
2099-2110.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.C.Hsieh,
S.A.Slater,
G.K.Whitfield,
J.L.Dawson,
G.Hsieh,
C.Sheedy,
C.A.Haussler,
and
M.R.Haussler
(2010).
Analysis of hairless corepressor mutants to characterize molecular cooperation with the vitamin D receptor in promoting the mammalian hair cycle.
|
| |
J Cell Biochem,
110,
671-686.
|
 |
|
|
|
|
 |
X.Hong,
J.Zang,
J.White,
C.Wang,
C.H.Pan,
R.Zhao,
R.C.Murphy,
S.Dai,
P.Henson,
J.W.Kappler,
J.Hagman,
and
G.Zhang
(2010).
Interaction of JMJD6 with single-stranded RNA.
|
| |
Proc Natl Acad Sci U S A,
107,
14568-14572.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Chen,
and
M.Costa
(2009).
Iron- and 2-oxoglutarate-dependent dioxygenases: an emerging group of molecular targets for nickel toxicity and carcinogenicity.
|
| |
Biometals,
22,
191-196.
|
 |
|
|
|
|
 |
M.Okamoto,
M.Van Stry,
L.Chung,
M.Koyanagi,
X.Sun,
Y.Suzuki,
O.Ohara,
H.Kitamura,
A.Hijikata,
M.Kubo,
and
M.Bix
(2009).
Mina, an Il4 repressor, controls T helper type 2 bias.
|
| |
Nat Immunol,
10,
872-879.
|
 |
|
|
|
|
 |
T.Sakamoto,
and
M.Seiki
(2009).
Mint3 enhances the activity of hypoxia-inducible factor-1 (HIF-1) in macrophages by suppressing the activity of factor inhibiting HIF-1.
|
| |
J Biol Chem,
284,
30350-30359.
|
 |
|
|
|
|
 |
B.Lohkamp,
and
D.Dobritzsch
(2008).
A mixture of fortunes: the curious determination of the structure of Escherichia coli BL21 Gab protein.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
407-415.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.H.Shin,
Y.S.Chun,
D.S.Lee,
L.E.Huang,
and
J.W.Park
(2008).
Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1.
|
| |
Blood,
111,
3131-3136.
|
 |
|
|
|
|
 |
J.M.Simmons,
T.A.Müller,
and
R.P.Hausinger
(2008).
Fe(II)/alpha-ketoglutarate hydroxylases involved in nucleobase, nucleoside, nucleotide, and chromatin metabolism.
|
| |
Dalton Trans,
(),
5132-5142.
|
 |
|
|
|
|
 |
P.Hahn,
J.Böse,
S.Edler,
and
A.Lengeling
(2008).
Genomic structure and expression of Jmjd6 and evolutionary analysis in the context of related JmjC domain containing proteins.
|
| |
BMC Genomics,
9,
293.
|
 |
|
|
|
|
 |
R.Chowdhury,
A.Hardy,
and
C.J.Schofield
(2008).
The human oxygen sensing machinery and its manipulation.
|
| |
Chem Soc Rev,
37,
1308-1319.
|
 |
|
|
|
|
 |
Y.H.Chen,
L.M.Comeaux,
R.W.Herbst,
E.Saban,
D.C.Kennedy,
M.J.Maroney,
and
M.J.Knapp
(2008).
Coordination changes and auto-hydroxylation of FIH-1: uncoupled O2-activation in a human hypoxia sensor.
|
| |
J Inorg Biochem,
102,
2120-2129.
|
 |
|
|
|
|
 |
A.B.Johnson,
and
M.C.Barton
(2007).
Hypoxia-induced and stress-specific changes in chromatin structure and function.
|
| |
Mutat Res,
618,
149-162.
|
 |
|
|
|
|
 |
A.Ozer,
and
R.K.Bruick
(2007).
Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one?
|
| |
Nat Chem Biol,
3,
144-153.
|
 |
|
|
|
|
 |
B.Chang,
Y.Chen,
Y.Zhao,
and
R.K.Bruick
(2007).
JMJD6 is a histone arginine demethylase.
|
| |
Science,
318,
444-447.
|
 |
|
|
|
|
 |
J.Li,
E.Wang,
S.Dutta,
J.S.Lau,
S.W.Jiang,
K.Datta,
and
D.Mukhopadhyay
(2007).
Protein kinase C-mediated modulation of FIH-1 expression by the homeodomain protein CDP/Cut/Cux.
|
| |
Mol Cell Biol,
27,
7345-7353.
|
 |
|
|
|
|
 |
Q.Yan,
S.Bartz,
M.Mao,
L.Li,
and
W.G.Kaelin
(2007).
The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo.
|
| |
Mol Cell Biol,
27,
2092-2102.
|
 |
|
|
|
|
 |
V.Purpero,
and
G.R.Moran
(2007).
The diverse and pervasive chemistries of the alpha-keto acid dependent enzymes.
|
| |
J Biol Inorg Chem,
12,
587-601.
|
 |
|
|
|
|
 |
J.L.Anderson,
and
S.K.Chapman
(2006).
Molecular mechanisms of enzyme-catalysed halogenation.
|
| |
Mol Biosyst,
2,
350-357.
|
 |
|
|
|
|
 |
K.D.Koehntop,
S.Marimanikkuppam,
M.J.Ryle,
R.P.Hausinger,
and
L.Que
(2006).
Self-hydroxylation of taurine/alpha-ketoglutarate dioxygenase: evidence for more than one oxygen activation mechanism.
|
| |
J Biol Inorg Chem,
11,
63-72.
|
 |
|
|
|
|
 |
L.C.Blasiak,
F.H.Vaillancourt,
C.T.Walsh,
and
C.L.Drennan
(2006).
Crystal structure of the non-haem iron halogenase SyrB2 in syringomycin biosynthesis.
|
| |
Nature,
440,
368-371.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.A.McDonough,
V.Li,
E.Flashman,
R.Chowdhury,
C.Mohr,
B.M.Liénard,
J.Zondlo,
N.J.Oldham,
I.J.Clifton,
J.Lewis,
L.A.McNeill,
R.J.Kurzeja,
K.S.Hewitson,
E.Yang,
S.Jordan,
R.S.Syed,
and
C.J.Schofield
(2006).
Cellular oxygen sensing: Crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2).
|
| |
Proc Natl Acad Sci U S A,
103,
9814-9819.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Zofall,
and
S.I.Grewal
(2006).
Swi6/HP1 recruits a JmjC domain protein to facilitate transcription of heterochromatic repeats.
|
| |
Mol Cell,
22,
681-692.
|
 |
|
|
|
|
 |
R.J.Klose,
E.M.Kallin,
and
Y.Zhang
(2006).
JmjC-domain-containing proteins and histone demethylation.
|
| |
Nat Rev Genet,
7,
715-727.
|
 |
|
|
|
|
 |
T.A.Müller,
M.I.Zavodszky,
M.Feig,
L.A.Kuhn,
and
R.P.Hausinger
(2006).
Structural basis for the enantiospecificities of R- and S-specific phenoxypropionate/alpha-ketoglutarate dioxygenases.
|
| |
Protein Sci,
15,
1356-1368.
|
 |
|
|
|
|
 |
H.J.Dyson,
and
P.E.Wright
(2005).
Intrinsically unstructured proteins and their functions.
|
| |
Nat Rev Mol Cell Biol,
6,
197-208.
|
 |
|
|
|
|
 |
C.J.Schofield,
and
P.J.Ratcliffe
(2004).
Oxygen sensing by HIF hydroxylases.
|
| |
Nat Rev Mol Cell Biol,
5,
343-354.
|
 |
|
|
|
|
 |
E.Metzen,
and
P.J.Ratcliffe
(2004).
HIF hydroxylation and cellular oxygen sensing.
|
| |
Biol Chem,
385,
223-230.
|
 |
|
|
|
|
 |
J.H.Distler,
R.H.Wenger,
M.Gassmann,
M.Kurowska,
A.Hirth,
S.Gay,
and
O.Distler
(2004).
Physiologic responses to hypoxia and implications for hypoxia-inducible factors in the pathogenesis of rheumatoid arthritis.
|
| |
Arthritis Rheum,
50,
10-23.
|
 |
|
|
|
|
 |
K.Ginalski,
L.Rychlewski,
D.Baker,
and
N.V.Grishin
(2004).
Protein structure prediction for the male-specific region of the human Y chromosome.
|
| |
Proc Natl Acad Sci U S A,
101,
2305-2310.
|
 |
|
|
|
|
 |
K.S.Hewitson,
and
C.J.Schofield
(2004).
The HIF pathway as a therapeutic target.
|
| |
Drug Discov Today,
9,
704-711.
|
 |
|
|
|
|
 |
K.Valegård,
A.C.Terwisscha van Scheltinga,
A.Dubus,
G.Ranghino,
L.M.Oster,
J.Hajdu,
and
I.Andersson
(2004).
The structural basis of cephalosporin formation in a mononuclear ferrous enzyme.
|
| |
Nat Struct Mol Biol,
11,
95.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Cikala,
O.Alexandrova,
C.N.David,
M.Pröschel,
B.Stiening,
P.Cramer,
and
A.Böttger
(2004).
The phosphatidylserine receptor from Hydra is a nuclear protein with potential Fe(II) dependent oxygenase activity.
|
| |
BMC Cell Biol,
5,
26.
|
 |
|
|
|
|
 |
W.Jelkmann
(2004).
Molecular biology of erythropoietin.
|
| |
Intern Med,
43,
649-659.
|
 |
|
|
|
|
 |
Z.Zhang,
J.S.Ren,
I.J.Clifton,
and
C.J.Schofield
(2004).
Crystal structure and mechanistic implications of 1-aminocyclopropane-1-carboxylic acid oxidase--the ethylene-forming enzyme.
|
| |
Chem Biol,
11,
1383-1394.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Goda,
S.J.Dozier,
and
R.S.Johnson
(2003).
HIF-1 in cell cycle regulation, apoptosis, and tumor progression.
|
| |
Antioxid Redox Signal,
5,
467-473.
|
 |
|
|
|
|
 |
S.Bhattacharya,
and
P.J.Ratcliffe
(2003).
ExCITED about HIF.
|
| |
Nat Struct Biol,
10,
501-503.
|
 |
|
|
|
|
 |
S.J.Freedman,
Z.Y.Sun,
A.L.Kung,
D.S.France,
G.Wagner,
and
M.J.Eck
(2003).
Structural basis for negative regulation of hypoxia-inducible factor-1alpha by CITED2.
|
| |
Nat Struct Biol,
10,
504-512.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

