 |
PDBsum entry 1kvx

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
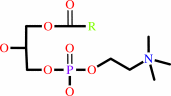 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Acta Crystallogr D Biol Crystallogr
55:443-447
(1999)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structures of the catalytic site mutants D99A and H48Q and the calcium-loop mutant D49E of phospholipase A2.
|
|
K.Sekar,
R.Biswas,
Y.Li,
M.Tsai,
M.Sundaralingam.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Crystal structures of the active-site mutants D99A and H48Q and the calcium-loop
mutant D49E of bovine phospholipase A2 have been determined at around 1.9 A
resolution. The D99A mutant is isomorphous to the orthorhombic recombinant
enzyme, space group P212121. The H48Q and the calcium-loop mutant D49E are
isomorphous to the trigonal recombinant enzyme, space group P3121. The two
active-site mutants show no major structural perturbations. The structural water
is absent in D99A and, therefore, the hydrogen-bonding scheme is changed. In
H48Q, the catalytic water is present and hydrogen bonded to Gln48 N, but the
second water found in native His48 is absent. In the calcium-loop mutant D49E,
the two water molecules forming the pentagonal bipyramid around calcium are
absent and only one O atom of the Glu49 carboxylate group is coordinated to
calcium, resulting in only four ligands.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3 The same stereoview of the calcium coordination in (a)
the mutant D49E and (b) in the trigonal recombinant PLA2 (Sekar
et al., 1998[Sekar, K., Sekharudu, Y. C., Tsai, M.-D. &
Sundaralingam, M. (1998). Acta Cryst. D54, 342-346.]). The
calcium ion is shown as a solid circle. The equatorial calcium
water W5 and the axial calcium water W12 are missing in the
mutant and are shown as open circles. In the mutant, only one of
the carboxylate O atoms is liganded to calcium, compared with
the recombinant enzyme where Asp49 forms a bidentate ligation.
Thus, calcium has only four ligands in the mutant while it has
seven ligands in the recombinant enzyme.
|
 |
Figure 4.
Figure 4 The omit electron density of the mutated residue Glu49
and the calcium ion. Contours are shown at the 1.0  level.
Note that there are only four ligands around the calcium ion. level.
Note that there are only four ligands around the calcium ion.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the IUCr:
Acta Crystallogr D Biol Crystallogr
(1999,
55,
443-447)
copyright 1999.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
 Google scholar
Google scholar
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.O.Kim,
B.K.Chakrabarti,
A.Guha-Niyogi,
M.K.Louder,
J.R.Mascola,
L.Ganesh,
and
G.J.Nabel
(2007).
Lysis of human immunodeficiency virus type 1 by a specific secreted human phospholipase A2.
|
| |
J Virol,
81,
1444-1450.
|
 |
|
|
|
|
 |
M.Rouault,
L.D.Rash,
P.Escoubas,
E.Boilard,
J.Bollinger,
B.Lomonte,
T.Maurin,
C.Guillaume,
S.Canaan,
C.Deregnaucourt,
J.Schrével,
A.Doglio,
J.M.Gutiérrez,
M.Lazdunski,
M.H.Gelb,
and
G.Lambeau
(2006).
Neurotoxicity and other pharmacological activities of the snake venom phospholipase A2 OS2: the N-terminal region is more important than enzymatic activity.
|
| |
Biochemistry,
45,
5800-5816.
|
 |
|
|
|
|
 |
K.Sekar,
V.Rajakannan,
D.Gayathri,
D.Velmurugan,
M.J.Poi,
M.Dauter,
Z.Dauter,
and
M.D.Tsai
(2005).
Atomic resolution (0.97 A) structure of the triple mutant (K53,56,121M) of bovine pancreatic phospholipase A2.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
61,
3-7.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.H.Gelb,
W.Cho,
and
D.C.Wilton
(1999).
Interfacial binding of secreted phospholipases A(2): more than electrostatics and a major role for tryptophan.
|
| |
Curr Opin Struct Biol,
9,
428-432.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

