 |
PDBsum entry 1k8c

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1k8c
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.1.1.307
- D-xylose reductase [NAD(P)H].
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
xylitol + NAD+ = D-xylose + NADH + H+
|
|
2.
|
xylitol + NADP+ = D-xylose + NADPH + H+
|
|
 |
 |
 |
 |
 |
xylitol
Bound ligand (Het Group name = )
matches with 91.67% similarity
|
+
|
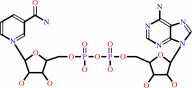 NAD(+)
NAD(+)
|
=
|
 D-xylose
D-xylose
|
+
|
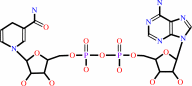 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
xylitol
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 NADP(+)
NADP(+)
|
=
|
 D-xylose
D-xylose
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
41:8785-8795
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
The structure of apo and holo forms of xylose reductase, a dimeric aldo-keto reductase from Candida tenuis.
|
|
K.L.Kavanagh,
M.Klimacek,
B.Nidetzky,
D.K.Wilson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Xylose reductase is a homodimeric oxidoreductase dependent on NADPH or NADH and
belongs to the largely monomeric aldo-keto reductase superfamily of proteins. It
catalyzes the first step in the assimilation of xylose, an aldose found to be a
major constituent monosaccharide of renewable plant hemicellulosic material,
into yeast metabolic pathways. It does this by reducing open chain xylose to
xylitol, which is reoxidized to xylulose by xylitol dehydrogenase and
metabolically integrated via the pentose phosphate pathway. No structure has yet
been determined for a xylose reductase, a dimeric aldo-keto reductase or a
family 2 aldo-keto reductase. The structures of the Candida tenuis xylose
reductase apo- and holoenzyme, which crystallize in spacegroup C2 with different
unit cells, have been determined to 2.2 A resolution and an R-factor of 17.9 and
20.8%, respectively. Residues responsible for mediating the novel dimeric
interface include Asp-178, Arg-181, Lys-202, Phe-206, Trp-313, and Pro-319.
Alignments with other superfamily members indicate that these interactions are
conserved in other dimeric xylose reductases but not throughout the remainder of
the oligomeric aldo-keto reductases, predicting alternate modes of
oligomerization for other families. An arrangement of side chains in a catalytic
triad shows that Tyr-52 has a conserved function as a general acid. The loop
that folds over the NAD(P)H cosubstrate is disordered in the apo form but
becomes ordered upon cosubstrate binding. A slow conformational isomerization of
this loop probably accounts for the observed rate-limiting step involving
release of cosubstrate. Xylose binding (K(m) = 87 mM) is mediated by
interactions with a binding pocket that is more polar than a typical aldo-keto
reductase. Modeling of xylose into the active site of the holoenzyme using
ordered waters as a guide for sugar hydroxyls suggests a convincing mode of
substrate binding.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Matsushika,
H.Inoue,
T.Kodaki,
and
S.Sawayama
(2009).
Ethanol production from xylose in engineered Saccharomyces cerevisiae strains: current state and perspectives.
|
| |
Appl Microbiol Biotechnol,
84,
37-53.
|
 |
|
|
|
|
 |
F.Zhang,
D.Qiao,
H.Xu,
C.Liao,
S.Li,
and
Y.Cao
(2009).
Cloning, expression, and characterization of xylose reductase with higher activity from Candida tropicalis.
|
| |
J Microbiol,
47,
351-357.
|
 |
|
|
|
|
 |
G.A.Khoury,
H.Fazelinia,
J.W.Chin,
R.J.Pantazes,
P.C.Cirino,
and
C.D.Maranas
(2009).
Computational design of Candida boidinii xylose reductase for altered cofactor specificity.
|
| |
Protein Sci,
18,
2125-2138.
|
 |
|
|
|
|
 |
L.C.Chen,
S.C.Huang,
P.Chuankhayan,
C.D.Chen,
Y.C.Huang,
J.Jeyakanthan,
H.F.Pang,
L.C.Men,
Y.C.Chen,
Y.K.Wang,
M.Y.Liu,
T.K.Wu,
and
C.J.Chen
(2009).
Purification, crystallization and preliminary X-ray crystallographic analysis of xylose reductase from Candida tropicalis.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
65,
419-421.
|
 |
|
|
|
|
 |
S.L.Pival,
M.Klimacek,
and
B.Nidetzky
(2009).
The catalytic mechanism of NADH-dependent reduction of 9,10-phenanthrenequinone by Candida tenuis xylose reductase reveals plasticity in an aldo-keto reductase active site.
|
| |
Biochem J,
421,
43-49.
|
 |
|
|
|
|
 |
A.Matsushika,
S.Watanabe,
T.Kodaki,
K.Makino,
H.Inoue,
K.Murakami,
O.Takimura,
and
S.Sawayama
(2008).
Expression of protein engineered NADP+-dependent xylitol dehydrogenase increases ethanol production from xylose in recombinant Saccharomyces cerevisiae.
|
| |
Appl Microbiol Biotechnol,
81,
243-255.
|
 |
|
|
|
|
 |
B.Petschacher,
and
B.Nidetzky
(2008).
Altering the coenzyme preference of xylose reductase to favor utilization of NADH enhances ethanol yield from xylose in a metabolically engineered strain of Saccharomyces cerevisiae.
|
| |
Microb Cell Fact,
7,
9.
|
 |
|
|
|
|
 |
J.G.Olsen,
L.Pedersen,
C.L.Christensen,
O.Olsen,
and
A.Henriksen
(2008).
Barley aldose reductase: structure, cofactor binding, and substrate recognition in the aldo/keto reductase 4C family.
|
| |
Proteins,
71,
1572-1581.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Puttick,
C.Vieille,
S.H.Song,
M.N.Fodje,
P.Grochulski,
and
L.T.Delbaere
(2007).
Crystallization, preliminary X-ray diffraction and structure analysis of Thermotoga maritima mannitol dehydrogenase.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
63,
350-352.
|
 |
|
|
|
|
 |
L.Liang,
J.Zhang,
and
Z.Lin
(2007).
Altering coenzyme specificity of Pichia stipitis xylose reductase by the semi-rational approach CASTing.
|
| |
Microb Cell Fact,
6,
36.
|
 |
|
|
|
|
 |
R.Kratzer,
and
B.Nidetzky
(2007).
Identification of Candida tenuis xylose reductase as highly selective biocatalyst for the synthesis of aromatic alpha-hydroxy esters and improvement of its efficiency by protein engineering.
|
| |
Chem Commun (Camb),
(),
1047-1049.
|
 |
|
|
|
|
 |
B.C.Chu,
and
H.Lee
(2006).
Investigation of the role of a conserved glycine motif in the Saccharomyces cerevisiae xylose reductase.
|
| |
Curr Microbiol,
53,
118-123.
|
 |
|
|
|
|
 |
C.Rosenthal,
U.Mueller,
S.Panjikar,
L.Sun,
M.Ruppert,
Y.Zhao,
and
J.Stöckigt
(2006).
Expression, purification, crystallization and preliminary X-ray analysis of perakine reductase, a new member of the aldo-keto reductase enzyme superfamily from higher plants.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
1286-1289.
|
 |
|
|
|
|
 |
J.F.Couture,
K.P.de Jésus-Tran,
A.M.Roy,
L.Cantin,
P.L.Côté,
P.Legrand,
V.Luu-The,
F.Labrie,
and
R.Breton
(2005).
Comparison of crystal structures of human type 3 3alpha-hydroxysteroid dehydrogenase reveals an "induced-fit" mechanism and a conserved basic motif involved in the binding of androgen.
|
| |
Protein Sci,
14,
1485-1497.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Ko,
I.Kim,
S.Yoo,
B.Min,
K.Kim,
and
C.Park
(2005).
Conversion of methylglyoxal to acetol by Escherichia coli aldo-keto reductases.
|
| |
J Bacteriol,
187,
5782-5789.
|
 |
|
|
|
|
 |
R.Woodyer,
M.Simurdiak,
W.A.van der Donk,
and
H.Zhao
(2005).
Heterologous expression, purification, and characterization of a highly active xylose reductase from Neurospora crassa.
|
| |
Appl Environ Microbiol,
71,
1642-1647.
|
 |
|
|
|
|
 |
G.Sanli,
S.Banta,
S.Anderson,
and
M.Blaber
(2004).
Structural alteration of cofactor specificity in Corynebacterium 2,5-diketo-D-gluconic acid reductase.
|
| |
Protein Sci,
13,
504-512.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
N.Yokochi,
Y.Yoshikane,
T.Yagi,
M.Yamasaki,
and
B.Mikami
(2004).
Crystallization and preliminary X-ray analysis of pyridoxal 4-dehydrogenase, the second enzyme in degradation pathway I of pyridoxine.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
2061-2062.
|
 |
|
|
|
|
 |
N.Yokochi,
Y.Yoshikane,
Y.Trongpanich,
K.Ohnishi,
and
T.Yagi
(2004).
Molecular cloning, expression, and properties of an unusual aldo-keto reductase family enzyme, pyridoxal 4-dehydrogenase, that catalyzes irreversible oxidation of pyridoxal.
|
| |
J Biol Chem,
279,
37377-37384.
|
 |
|
|
|
|
 |
A.Ehrensberger,
and
D.K.Wilson
(2003).
Expression, crystallization and activities of the two family 11 aldo-keto reductases from Bacillus subtilis.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
375-377.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

