 |
PDBsum entry 1k4j

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.3.1.184
- acyl-homoserine-lactone synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a fatty acyl-[ACP] + S-adenosyl-L-methionine = an N-acyl-L-homoserine lactone + S-methyl-5'-thioadenosine + holo-[ACP] + H+
|
 |
 |
 |
 |
 |
fatty acyl-[ACP]
|
+
|
 S-adenosyl-L-methionine
S-adenosyl-L-methionine
|
=
|
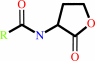 N-acyl-L-homoserine lactone
N-acyl-L-homoserine lactone
|
+
|
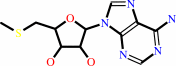 S-methyl-5'-thioadenosine
S-methyl-5'-thioadenosine
|
+
|
holo-[ACP]
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Mol Cell
9:685-694
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing.
|
|
W.T.Watson,
T.D.Minogue,
D.L.Val,
S.B.von Bodman,
M.E.Churchill.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Synthesis and detection of acyl-homoserine lactones (AHLs) enables many
gram-negative bacteria to engage in quorum sensing, an intercellular signaling
mechanism that activates differentiation to virulent and biofilm lifestyles. The
AHL synthases catalyze acylation of S-adenosyl-L-methionine by acyl-acyl carrier
protein and lactonization of the methionine moiety to give AHLs. The crystal
structure of the AHL synthase, EsaI, determined at 1.8 A resolution, reveals a
remarkable structural similarity to the N-acetyltransferases and defines a
common phosphopantetheine binding fold as the catalytic core. Critical residues
responsible for catalysis and acyl chain specificity have been identified from a
modeled substrate complex and verified through functional analysis in vivo. A
mechanism for the N-acylation of S-adenosyl-L-methionine by 3-oxo-hexanoyl-acyl
carrier protein is proposed.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2. Sequence and Structural Alignment of Selected AHL
Synthases and GNATsThe sequence and topology of the AHL synthase
family is compared to the GCN5-related N-acetyltransferases. The
gray shaded regions are conserved sequence blocks within each
family that constitute the enzyme's “sequence signature.”
Residues are colored red to indicate acidic or hydrophilic, blue
for basic, and orange for other. Shaded residues are absolutely
conserved, and the boxed residues are homologous within each
family. Residues that comprise the core “phosphopantetheine
binding fold” were identified by LSQMAN using a 2.0 Å
cutoff and are indicated by black bars above the segments. The
Tetrahymena GCN5 residues that contact the pantetheine or acetyl
portion of the acetyl-CoA are indicated by “p” or “a,”
respectively.
|
 |
Figure 4.
Figure 4. Proposed Mechanism of Acyl Transfer(A) The
stereodiagram of acyl-phosphopantetheine modeled into the EsaI
active-site cavity viewed as in Figure 2A. The electrostatic
surface, generated using GRASP (Nicholls et al., 1993) and
Photoshop (Adobe), is colored red, white, and blue to indicate
negatively charged, neutral, or positively charged regions of
the surface, respectively. The individual atoms in the modeled
phosphopantetheine are colored according to atom type.(B) The
acylation cleft of EsaI and relevant residues are shown in
gray, the modeled phosphopanteteine is shown in cyan, and the
well-ordered water molecules observed in the native structure
that lie along β4 are shown as red spheres.(C) The proposed
N-acylation reaction is catalyzed via nucleophilic attack on the
1-carbonyl of acyl-ACP by the free amine electrons of SAM after
proton abstraction by a water molecule stabilized by Glu97 or
Ser99.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Cell Press:
Mol Cell
(2002,
9,
685-694)
copyright 2002.
|
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.Hothersall,
A.C.Murphy,
Z.Iqbal,
G.Campbell,
E.R.Stephens,
J.Wu,
H.Cooper,
S.Atkinson,
P.Williams,
J.Crosby,
C.L.Willis,
R.J.Cox,
T.J.Simpson,
and
C.M.Thomas
(2011).
Manipulation of quorum sensing regulation in Pseudomonas fluorescens NCIMB 10586 to increase mupirocin production.
|
| |
Appl Microbiol Biotechnol,
90,
1017-1026.
|
 |
|
|
|
|
 |
V.C.Kalia,
and
H.J.Purohit
(2011).
Quenching the quorum sensing system: potential antibacterial drug targets.
|
| |
Crit Rev Microbiol,
37,
121-140.
|
 |
|
|
|
|
 |
J.S.Dickschat
(2010).
Quorum sensing and bacterial biofilms.
|
| |
Nat Prod Rep,
27,
343-369.
|
 |
|
|
|
|
 |
M.Tizzano,
B.D.Gulbransen,
A.Vandenbeuch,
T.R.Clapp,
J.P.Herman,
H.M.Sibhatu,
M.E.Churchill,
W.L.Silver,
S.C.Kinnamon,
and
T.E.Finger
(2010).
Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals.
|
| |
Proc Natl Acad Sci U S A,
107,
3210-3215.
|
 |
|
|
|
|
 |
P.K.Kambam,
D.T.Eriksen,
J.Lajoie,
D.J.Sayut,
and
L.Sun
(2009).
Altering the substrate specificity of RhlI by directed evolution.
|
| |
Chembiochem,
10,
553-558.
|
 |
|
|
|
|
 |
R.J.Malott,
E.P.O'Grady,
J.Toller,
S.Inhülsen,
L.Eberl,
and
P.A.Sokol
(2009).
A Burkholderia cenocepacia orphan LuxR homolog is involved in quorum-sensing regulation.
|
| |
J Bacteriol,
191,
2447-2460.
|
 |
|
|
|
|
 |
T.R.Cataldi,
G.Bianco,
S.Abate,
and
D.Mattia
(2009).
Analysis of S-adenosylmethionine and related sulfur metabolites in bacterial isolates of Pseudomonas aeruginosa (BAA-47) by liquid chromatography/electrospray ionization coupled to a hybrid linear quadrupole ion trap and Fourier transform ion cyclotron resonance mass spectrometry.
|
| |
Rapid Commun Mass Spectrom,
23,
3465-3477.
|
 |
|
|
|
|
 |
W.L.Ng,
and
B.L.Bassler
(2009).
Bacterial quorum-sensing network architectures.
|
| |
Annu Rev Genet,
43,
197-222.
|
 |
|
|
|
|
 |
A.Jayaraman,
and
T.K.Wood
(2008).
Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease.
|
| |
Annu Rev Biomed Eng,
10,
145-167.
|
 |
|
|
|
|
 |
J.M.Norton,
M.G.Klotz,
L.Y.Stein,
D.J.Arp,
P.J.Bottomley,
P.S.Chain,
L.J.Hauser,
M.L.Land,
F.W.Larimer,
M.W.Shin,
and
S.R.Starkenburg
(2008).
Complete genome sequence of Nitrosospira multiformis, an ammonia-oxidizing bacterium from the soil environment.
|
| |
Appl Environ Microbiol,
74,
3559-3572.
|
 |
|
|
|
|
 |
J.S.Cisar,
and
D.S.Tan
(2008).
Small molecule inhibition of microbial natural product biosynthesis-an emerging antibiotic strategy.
|
| |
Chem Soc Rev,
37,
1320-1329.
|
 |
|
|
|
|
 |
M.Cooley,
S.R.Chhabra,
and
P.Williams
(2008).
N-Acylhomoserine lactone-mediated quorum sensing: a twist in the tail and a blow for host immunity.
|
| |
Chem Biol,
15,
1141-1147.
|
 |
|
|
|
|
 |
P.K.Kambam,
D.J.Sayut,
Y.Niu,
D.T.Eriksen,
and
L.Sun
(2008).
Directed evolution of LuxI for enhanced OHHL production.
|
| |
Biotechnol Bioeng,
101,
263-272.
|
 |
|
|
|
|
 |
A.M.Barnard,
S.D.Bowden,
T.Burr,
S.J.Coulthurst,
R.E.Monson,
and
G.P.Salmond
(2007).
Quorum sensing, virulence and secondary metabolite production in plant soft-rotting bacteria.
|
| |
Philos Trans R Soc Lond B Biol Sci,
362,
1165-1183.
|
 |
|
|
|
|
 |
C.E.White,
and
S.C.Winans
(2007).
Cell-cell communication in the plant pathogen Agrobacterium tumefaciens.
|
| |
Philos Trans R Soc Lond B Biol Sci,
362,
1135-1148.
|
 |
|
|
|
|
 |
P.Williams,
K.Winzer,
W.C.Chan,
and
M.Cámara
(2007).
Look who's talking: communication and quorum sensing in the bacterial world.
|
| |
Philos Trans R Soc Lond B Biol Sci,
362,
1119-1134.
|
 |
|
|
|
|
 |
R.Van Houdt,
M.Givskov,
and
C.W.Michiels
(2007).
Quorum sensing in Serratia.
|
| |
FEMS Microbiol Rev,
31,
407-424.
|
 |
|
|
|
|
 |
T.P.Huang,
and
A.C.Wong
(2007).
A cyclic AMP receptor protein-regulated cell-cell communication system mediates expression of a FecA homologue in Stenotrophomonas maltophilia.
|
| |
Appl Environ Microbiol,
73,
5034-5040.
|
 |
|
|
|
|
 |
W.Nasser,
and
S.Reverchon
(2007).
New insights into the regulatory mechanisms of the LuxR family of quorum sensing regulators.
|
| |
Anal Bioanal Chem,
387,
381-390.
|
 |
|
|
|
|
 |
A.L.Carlier,
and
S.B.von Bodman
(2006).
The rcsA promoter of Pantoea stewartii subsp. stewartii features a low-level constitutive promoter and an EsaR quorum-sensing-regulated promoter.
|
| |
J Bacteriol,
188,
4581-4584.
|
 |
|
|
|
|
 |
C.G.Penalver,
F.Cantet,
D.Morin,
D.Haras,
and
J.A.Vorholt
(2006).
A plasmid-borne truncated luxI homolog controls quorum-sensing systems and extracellular carbohydrate production in Methylobacterium extorquens AM1.
|
| |
J Bacteriol,
188,
7321-7324.
|
 |
|
|
|
|
 |
G.Hao,
and
T.J.Burr
(2006).
Regulation of long-chain N-acyl-homoserine lactones in Agrobacterium vitis.
|
| |
J Bacteriol,
188,
2173-2183.
|
 |
|
|
|
|
 |
H.Zheng,
Z.Zhong,
X.Lai,
W.X.Chen,
S.Li,
and
J.Zhu
(2006).
A LuxR/LuxI-type quorum-sensing system in a plant bacterium, Mesorhizobium tianshanense, controls symbiotic nodulation.
|
| |
J Bacteriol,
188,
1943-1949.
|
 |
|
|
|
|
 |
J.E.González,
and
N.D.Keshavan
(2006).
Messing with bacterial quorum sensing.
|
| |
Microbiol Mol Biol Rev,
70,
859-875.
|
 |
|
|
|
|
 |
M.D.Koutsoudis,
D.Tsaltas,
T.D.Minogue,
and
S.B.von Bodman
(2006).
Quorum-sensing regulation governs bacterial adhesion, biofilm development, and host colonization in Pantoea stewartii subspecies stewartii.
|
| |
Proc Natl Acad Sci U S A,
103,
5983-5988.
|
 |
|
|
|
|
 |
M.E.Churchill
(2006).
A new GNAT in bacterial signaling?
|
| |
Structure,
14,
1342-1344.
|
 |
|
|
|
|
 |
R.A.Scott,
J.Weil,
P.T.Le,
P.Williams,
R.G.Fray,
S.B.von Bodman,
and
M.A.Savka
(2006).
Long- and short-chain plant-produced bacterial N-acyl-homoserine lactones become components of phyllosphere, rhizosphere, and soil.
|
| |
Mol Plant Microbe Interact,
19,
227-239.
|
 |
|
|
|
|
 |
R.M.Van Wagoner,
and
J.Clardy
(2006).
FeeM, an N-acyl amino acid synthase from an uncultured soil microbe: structure, mechanism, and acyl carrier protein binding.
|
| |
Structure,
14,
1425-1435.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.A.Gould,
J.Herman,
J.Krank,
R.C.Murphy,
and
M.E.Churchill
(2006).
Specificity of acyl-homoserine lactone synthases examined by mass spectrometry.
|
| |
J Bacteriol,
188,
773-783.
|
 |
|
|
|
|
 |
C.Farah,
M.Vera,
D.Morin,
D.Haras,
C.A.Jerez,
and
N.Guiliani
(2005).
Evidence for a functional quorum-sensing type AI-1 system in the extremophilic bacterium Acidithiobacillus ferrooxidans.
|
| |
Appl Environ Microbiol,
71,
7033-7040.
|
 |
|
|
|
|
 |
C.M.Waters,
and
B.L.Bassler
(2005).
Quorum sensing: cell-to-cell communication in bacteria.
|
| |
Annu Rev Cell Dev Biol,
21,
319-346.
|
 |
|
|
|
|
 |
G.Brader,
S.Sjöblom,
H.Hyytiäinen,
K.Sims-Huopaniemi,
and
E.T.Palva
(2005).
Altering substrate chain length specificity of an acylhomoserine lactone synthase in bacterial communication.
|
| |
J Biol Chem,
280,
10403-10409.
|
 |
|
|
|
|
 |
M.Frommberger,
N.Hertkorn,
M.Englmann,
S.Jakoby,
A.Hartmann,
A.Kettrup,
and
P.Schmitt-Kopplin
(2005).
Analysis of N-acylhomoserine lactones after alkaline hydrolysis and anion-exchange solid-phase extraction by capillary zone electrophoresis-mass spectrometry.
|
| |
Electrophoresis,
26,
1523-1532.
|
 |
|
|
|
|
 |
M.H.Kim,
W.C.Choi,
H.O.Kang,
J.S.Lee,
B.S.Kang,
K.J.Kim,
Z.S.Derewenda,
T.K.Oh,
C.H.Lee,
and
J.K.Lee
(2005).
The molecular structure and catalytic mechanism of a quorum-quenching N-acyl-L-homoserine lactone hydrolase.
|
| |
Proc Natl Acad Sci U S A,
102,
17606-17611.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.Z.Kozbial,
and
A.R.Mushegian
(2005).
Natural history of S-adenosylmethionine-binding proteins.
|
| |
BMC Struct Biol,
5,
19.
|
 |
|
|
|
|
 |
S.R.Khan,
D.V.Mavrodi,
G.J.Jog,
H.Suga,
L.S.Thomashow,
and
S.K.Farrand
(2005).
Activation of the phz operon of Pseudomonas fluorescens 2-79 requires the LuxR homolog PhzR, N-(3-OH-Hexanoyl)-L-homoserine lactone produced by the LuxI homolog PhzI, and a cis-acting phz box.
|
| |
J Bacteriol,
187,
6517-6527.
|
 |
|
|
|
|
 |
J.A.Newton,
and
R.G.Fray
(2004).
Integration of environmental and host-derived signals with quorum sensing during plant-microbe interactions.
|
| |
Cell Microbiol,
6,
213-224.
|
 |
|
|
|
|
 |
K.M.Pappas,
C.L.Weingart,
and
S.C.Winans
(2004).
Chemical communication in proteobacteria: biochemical and structural studies of signal synthases and receptors required for intercellular signalling.
|
| |
Mol Microbiol,
53,
755-769.
|
 |
|
|
|
|
 |
R.Daniels,
J.Vanderleyden,
and
J.Michiels
(2004).
Quorum sensing and swarming migration in bacteria.
|
| |
FEMS Microbiol Rev,
28,
261-289.
|
 |
|
|
|
|
 |
S.F.Brady,
C.J.Chao,
and
J.Clardy
(2004).
Long-chain N-acyltyrosine synthases from environmental DNA.
|
| |
Appl Environ Microbiol,
70,
6865-6870.
|
 |
|
|
|
|
 |
T.A.Gould,
H.P.Schweizer,
and
M.E.Churchill
(2004).
Structure of the Pseudomonas aeruginosa acyl-homoserinelactone synthase LasI.
|
| |
Mol Microbiol,
53,
1135-1146.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.A.Gould,
W.T.Watson,
K.H.Choi,
H.P.Schweizer,
and
M.E.Churchill
(2004).
Crystallization of Pseudomonas aeruginosa AHL synthase LasI using beta-turn crystal engineering.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
518-520.
|
 |
|
|
|
|
 |
J.E.González,
and
M.M.Marketon
(2003).
Quorum sensing in nitrogen-fixing rhizobia.
|
| |
Microbiol Mol Biol Rev,
67,
574-592.
|
 |
|
|
|
|
 |
J.N.Smith,
and
B.M.Ahmer
(2003).
Detection of other microbial species by Salmonella: expression of the SdiA regulon.
|
| |
J Bacteriol,
185,
1357-1366.
|
 |
|
|
|
|
 |
L.H.Zhang
(2003).
Quorum quenching and proactive host defense.
|
| |
Trends Plant Sci,
8,
238-244.
|
 |
|
|
|
|
 |
M.E.Taga,
and
B.L.Bassler
(2003).
Chemical communication among bacteria.
|
| |
Proc Natl Acad Sci U S A,
100,
14549-14554.
|
 |
|
|
|
|
 |
M.Mitsumori,
L.Xu,
H.Kajikawa,
M.Kurihara,
K.Tajima,
J.Hai,
and
A.Takenaka
(2003).
Possible quorum sensing in the rumen microbial community: detection of quorum-sensing signal molecules from rumen bacteria.
|
| |
FEMS Microbiol Lett,
219,
47-52.
|
 |
|
|
|
|
 |
M.W.Vetting,
S.L.Roderick,
M.Yu,
and
J.S.Blanchard
(2003).
Crystal structure of mycothiol synthase (Rv0819) from Mycobacterium tuberculosis shows structural homology to the GNAT family of N-acetyltransferases.
|
| |
Protein Sci,
12,
1954-1959.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.B.Von Bodman,
W.D.Bauer,
and
D.L.Coplin
(2003).
Quorum sensing in plant-pathogenic bacteria.
|
| |
Annu Rev Phytopathol,
41,
455-482.
|
 |
|
|
|
|
 |
B.L.Bassler
(2002).
Small talk. Cell-to-cell communication in bacteria.
|
| |
Cell,
109,
421-424.
|
 |
|
|
|
|
 |
M.Cámara,
P.Williams,
and
A.Hardman
(2002).
Controlling infection by tuning in and turning down the volume of bacterial small-talk.
|
| |
Lancet Infect Dis,
2,
667-676.
|
 |
|
|
|
|
 |
P.Williams
(2002).
Quorum sensing: an emerging target for antibacterial chemotherapy?
|
| |
Expert Opin Ther Targets,
6,
257-274.
|
 |
|
|
|
|
 |
Y.T.Horng,
S.C.Deng,
M.Daykin,
P.C.Soo,
J.R.Wei,
K.T.Luh,
S.W.Ho,
S.Swift,
H.C.Lai,
and
P.Williams
(2002).
The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing in Serratia marcescens.
|
| |
Mol Microbiol,
45,
1655-1671.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

