 |
PDBsum entry 1j8f

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Gene regulation, transferase
|
PDB id
|
|

|

|
1j8f
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Gene regulation, transferase
|
 |
|
Title:
|
 |
Human sirt2 histone deacetylase
|
|
Structure:
|
 |
Sirtuin 2, isoform 1. Chain: a, b, c. Synonym: sirt2. Sir2-related protein type 2. Silencing information regulator 2-like. Sir2-like 2. Silent mating type information regulation 2, s.Cerevisiae, homolog 2. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: sirt2. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008.
|
|
Biol. unit:
|
 |
Dimer (from
)
|
|
Resolution:
|
 |
|
1.70Å
|
R-factor:
|
0.235
|
R-free:
|
0.260
|
|
|
Authors:
|
 |
N.P.Pavletich,M.S.Finnin,J.R.Donigian
|
Key ref:

|
 |
M.S.Finnin
et al.
(2001).
Structure of the histone deacetylase SIRT2.
Nat Struct Biol,
8,
621-625.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
21-May-01
|
Release date:
|
06-Jul-01
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q8IXJ6
(SIR2_HUMAN) -
NAD-dependent protein deacetylase sirtuin-2 from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
389 a.a.
312 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.3.1.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.3.1.286
- protein acetyllysine N-acetyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N6-acetyl-L-lysyl-[protein] + NAD+ + H2O = 2''-O-acetyl-ADP-D-ribose + nicotinamide + L-lysyl-[protein]
|
 |
 |
 |
 |
 |
N(6)-acetyl-L-lysyl-[protein]
|
+
|
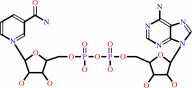 NAD(+)
NAD(+)
|
+
|
H2O
|
=
|
2''-O-acetyl-ADP-D-ribose
|
+
|
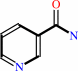 nicotinamide
nicotinamide
|
+
|
L-lysyl-[protein]
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nat Struct Biol
8:621-625
(2001)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of the histone deacetylase SIRT2.
|
|
M.S.Finnin,
J.R.Donigian,
N.P.Pavletich.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Sir2 is an NAD-dependent histone deacetylase that mediates transcriptional
silencing at mating-type loci, telomeres and ribosomal gene clusters, and has a
critical role in the determination of life span in yeast and Caenorhabditis
elegans. The 1.7 A crystal structure of the 323 amino acid catalytic core of
human SIRT2, a homolog of yeast Sir2, reveals an NAD-binding domain, which is a
variant of the Rossmann fold, and a smaller domain composed of a helical module
and a zinc-binding module. A conserved large groove at the interface of the two
domains is the likely site of catalysis based on mutagenesis. Intersecting this
large groove, there is a pocket formed by the helical module. The pocket is
lined with hydrophobic residues conserved within each of the five Sir2 classes,
suggesting that it is a class-specific protein-binding site.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Conservation among Sir2-like enzymes. a, Surface
representation of SIRT2 generated by the program GRASP39, with
residues identical among all Sir2-like enzymes (yellow) and
those identical among class I enzymes (magenta) mapped onto the
surface. These residues are indicated as ball-and-stick
representations in the black-rimmed expansion (right), which
shows a close-up view of the potential NAD-binding site. b, An
expanded surface representation of the area highlighted in (a)
with the hydrophobic residues of the pocket mapped onto the
surface in orange. The black-rimmed expansion shows a ribbon
diagram of the helical module containing the hydrophobic pocket
and ball-and-stick representation of the hydrophobic residues,
which are labeled. c, Histone deacetylase activity of the SIRT2
point mutants. Reactions contained wild type or mutant SIRT2,
500  M
NAD and 10 M
NAD and 10  g
of [3H]acetyl-labeled murine erythroleukemia histone
preparation. Assays were performed in triplicate and error bars
denote the standard deviation. g
of [3H]acetyl-labeled murine erythroleukemia histone
preparation. Assays were performed in triplicate and error bars
denote the standard deviation.
|
 |
Figure 4.
Figure 4. Structural comparison between SIRT2 and Sir2-Af1.
a, Superposition of SIRT2 Rossmann fold with Sir2-Af1 in stereo.
SIRT2 is shown in cyan and positioned approximately in the same
orientation as in Fig. 1 (right), and Sir2-Af1 is in gray. The
zinc from SIRT2 is in magenta, whereas the zinc atom from
Sir2-Af1 is in orange. The NAD molecule from the Sir2-Af1 -NAD
complex is in red. The SIRT2 small pocket and the L-1B loop of
Sir2-Af1 are labeled. Notice the increased size of the Sir2-Af1
acetyl-lysine binding site in SIRT2. b, A stereo, close-up view
of the NAD binding sites of SIRT2 and Sir2-Af1. The view shows
the molecules turned 90° along a horizontal axis in the plane of
the paper. Conserved SIRT2 residues that are candidates for
NAD-binding or catalysis are shown as ball-and-stick
representations and are labeled.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nat Struct Biol
(2001,
8,
621-625)
copyright 2001.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
P.Bheda,
J.T.Wang,
J.C.Escalante-Semerena,
and
C.Wolberger
(2011).
Structure of Sir2Tm bound to a propionylated peptide.
|
| |
Protein Sci,
20,
131-139.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.Verdin,
M.D.Hirschey,
L.W.Finley,
and
M.C.Haigis
(2010).
Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling.
|
| |
Trends Biochem Sci,
35,
669-675.
|
 |
|
|
|
|
 |
J.Schemies,
U.Uciechowska,
W.Sippl,
and
M.Jung
(2010).
NAD(+) -dependent histone deacetylases (sirtuins) as novel therapeutic targets.
|
| |
Med Res Rev,
30,
861-889.
|
 |
|
|
|
|
 |
J.Tavares,
A.Ouaissi,
P.Kong Thoo Lin,
I.Loureiro,
S.Kaur,
N.Roy,
and
A.Cordeiro-da-Silva
(2010).
Bisnaphthalimidopropyl derivatives as inhibitors of Leishmania SIR2 related protein 1.
|
| |
ChemMedChem,
5,
140-147.
|
 |
|
|
|
|
 |
M.A.Wouters,
S.W.Fan,
and
N.L.Haworth
(2010).
Disulfides as redox switches: from molecular mechanisms to functional significance.
|
| |
Antioxid Redox Signal,
12,
53-91.
|
 |
|
|
|
|
 |
S.Kaur,
A.V.Shivange,
and
N.Roy
(2010).
Structural analysis of trypanosomal sirtuin: an insight for selective drug design.
|
| |
Mol Divers,
14,
169-178.
|
 |
|
|
|
|
 |
A.Mai,
and
L.Altucci
(2009).
Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead.
|
| |
Int J Biochem Cell Biol,
41,
199-213.
|
 |
|
|
|
|
 |
D.Albani,
L.Polito,
S.Batelli,
S.De Mauro,
C.Fracasso,
G.Martelli,
L.Colombo,
C.Manzoni,
M.Salmona,
S.Caccia,
A.Negro,
and
G.Forloni
(2009).
The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide.
|
| |
J Neurochem,
110,
1445-1456.
|
 |
|
|
|
|
 |
D.Wang
(2009).
Computational studies on the histone deacetylases and the design of selective histone deacetylase inhibitors.
|
| |
Curr Top Med Chem,
9,
241-256.
|
 |
|
|
|
|
 |
D.Zhang,
S.Li,
P.Cruz,
and
B.C.Kone
(2009).
Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate alpha-ENaC transcription in collecting duct.
|
| |
J Biol Chem,
284,
20917-20926.
|
 |
|
|
|
|
 |
E.Lara,
A.Mai,
V.Calvanese,
L.Altucci,
P.Lopez-Nieva,
M.L.Martinez-Chantar,
M.Varela-Rey,
D.Rotili,
A.Nebbioso,
S.Ropero,
G.Montoya,
J.Oyarzabal,
S.Velasco,
M.Serrano,
M.Witt,
A.Villar-Garea,
A.Inhof,
J.M.Mato,
M.Esteller,
and
M.F.Fraga
(2009).
Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect.
|
| |
Oncogene,
28,
781-791.
|
 |
|
|
|
|
 |
F.Medda,
R.J.Russell,
M.Higgins,
A.R.McCarthy,
J.Campbell,
A.M.Slawin,
D.P.Lane,
S.Lain,
and
N.J.Westwood
(2009).
Novel cambinol analogs as sirtuin inhibitors: synthesis, biological evaluation, and rationalization of activity.
|
| |
J Med Chem,
52,
2673-2682.
|
 |
|
|
|
|
 |
F.Wang,
and
Q.Tong
(2009).
SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1's repressive interaction with PPARgamma.
|
| |
Mol Biol Cell,
20,
801-808.
|
 |
|
|
|
|
 |
K.S.Makarova,
Y.I.Wolf,
J.van der Oost,
and
E.V.Koonin
(2009).
Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements.
|
| |
Biol Direct,
4,
29.
|
 |
|
|
|
|
 |
L.Jin,
W.Wei,
Y.Jiang,
H.Peng,
J.Cai,
C.Mao,
H.Dai,
W.Choy,
J.E.Bemis,
M.R.Jirousek,
J.C.Milne,
C.H.Westphal,
and
R.B.Perni
(2009).
Crystal structures of human SIRT3 displaying substrate-induced conformational changes.
|
| |
J Biol Chem,
284,
24394-24405.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Suzuki
(2009).
Explorative study on isoform-selective histone deacetylase inhibitors.
|
| |
Chem Pharm Bull (Tokyo),
57,
897-906.
|
 |
|
|
|
|
 |
D.P.Dowling,
L.Di Costanzo,
H.A.Gennadios,
and
D.W.Christianson
(2008).
Evolution of the arginase fold and functional diversity.
|
| |
Cell Mol Life Sci,
65,
2039-2055.
|
 |
|
|
|
|
 |
E.G.Lynn,
C.J.McLeod,
J.P.Gordon,
J.Bao,
and
M.N.Sack
(2008).
SIRT2 is a negative regulator of anoxia-reoxygenation tolerance via regulation of 14-3-3 zeta and BAD in H9c2 cells.
|
| |
FEBS Lett,
582,
2857-2862.
|
 |
|
|
|
|
 |
I.Autiero,
S.Costantini,
and
G.Colonna
(2008).
Human sirt-1: molecular modeling and structure-function relationships of an unordered protein.
|
| |
PLoS One,
4,
e7350.
|
 |
|
|
|
|
 |
J.A.Kovacs,
M.Yeager,
and
R.Abagyan
(2008).
Damped-dynamics flexible fitting.
|
| |
Biophys J,
95,
3192-3207.
|
 |
|
|
|
|
 |
J.C.Milne,
and
J.M.Denu
(2008).
The Sirtuin family: therapeutic targets to treat diseases of aging.
|
| |
Curr Opin Chem Biol,
12,
11-17.
|
 |
|
|
|
|
 |
P.Hu,
S.Wang,
and
Y.Zhang
(2008).
Highly dissociative and concerted mechanism for the nicotinamide cleavage reaction in Sir2Tm enzyme suggested by ab initio QM/MM molecular dynamics simulations.
|
| |
J Am Chem Soc,
130,
16721-16728.
|
 |
|
|
|
|
 |
S.Lavu,
O.Boss,
P.J.Elliott,
and
P.D.Lambert
(2008).
Sirtuins--novel therapeutic targets to treat age-associated diseases.
|
| |
Nat Rev Drug Discov,
7,
841-853.
|
 |
|
|
|
|
 |
U.Uciechowska,
J.Schemies,
R.C.Neugebauer,
E.M.Huda,
M.L.Schmitt,
R.Meier,
E.Verdin,
M.Jung,
and
W.Sippl
(2008).
Thiobarbiturates as sirtuin inhibitors: virtual screening, free-energy calculations, and biological testing.
|
| |
ChemMedChem,
3,
1965-1976.
|
 |
|
|
|
|
 |
W.F.Hawse,
K.G.Hoff,
D.G.Fatkins,
A.Daines,
O.V.Zubkova,
V.L.Schramm,
W.Zheng,
and
C.Wolberger
(2008).
Structural insights into intermediate steps in the Sir2 deacetylation reaction.
|
| |
Structure,
16,
1368-1377.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Schuetz,
J.Min,
T.Antoshenko,
C.L.Wang,
A.Allali-Hassani,
A.Dong,
P.Loppnau,
M.Vedadi,
A.Bochkarev,
R.Sternglanz,
and
A.N.Plotnikov
(2007).
Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin.
|
| |
Structure,
15,
377-389.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.J.Merrick,
and
M.T.Duraisingh
(2007).
Plasmodium falciparum Sir2: an unusual sirtuin with dual histone deacetylase and ADP-ribosyltransferase activity.
|
| |
Eukaryot Cell,
6,
2081-2091.
|
 |
|
|
|
|
 |
F.Nahhas,
S.C.Dryden,
J.Abrams,
and
M.A.Tainsky
(2007).
Mutations in SIRT2 deacetylase which regulate enzymatic activity but not its interaction with HDAC6 and tubulin.
|
| |
Mol Cell Biochem,
303,
221-230.
|
 |
|
|
|
|
 |
F.Wang,
M.Nguyen,
F.X.Qin,
and
Q.Tong
(2007).
SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction.
|
| |
Aging Cell,
6,
505-514.
|
 |
|
|
|
|
 |
H.Yang,
J.A.Baur,
A.Chen,
C.Miller,
J.K.Adams,
A.Kisielewski,
K.T.Howitz,
R.E.Zipkin,
and
D.A.Sinclair
(2007).
Design and synthesis of compounds that extend yeast replicative lifespan.
|
| |
Aging Cell,
6,
35-43.
|
 |
|
|
|
|
 |
J.Mead,
R.McCord,
L.Youngster,
M.Sharma,
M.R.Gartenberg,
and
A.K.Vershon
(2007).
Swapping the gene-specific and regional silencing specificities of the Hst1 and Sir2 histone deacetylases.
|
| |
Mol Cell Biol,
27,
2466-2475.
|
 |
|
|
|
|
 |
J.Trapp,
R.Meier,
D.Hongwiset,
M.U.Kassack,
W.Sippl,
and
M.Jung
(2007).
Structure-Activity Studies on Suramin Analogues as Inhibitors of NAD(+)-Dependent Histone Deacetylases (Sirtuins).
|
| |
ChemMedChem,
2,
1419-1431.
|
 |
|
|
|
|
 |
S.Lall
(2007).
Primers on chromatin.
|
| |
Nat Struct Mol Biol,
14,
1110-1115.
|
 |
|
|
|
|
 |
T.Inoue,
M.Hiratsuka,
M.Osaki,
H.Yamada,
I.Kishimoto,
S.Yamaguchi,
S.Nakano,
M.Katoh,
H.Ito,
and
M.Oshimura
(2007).
SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress.
|
| |
Oncogene,
26,
945-957.
|
 |
|
|
|
|
 |
A.A.Sauve,
C.Wolberger,
V.L.Schramm,
and
J.D.Boeke
(2006).
The biochemistry of sirtuins.
|
| |
Annu Rev Biochem,
75,
435-465.
|
 |
|
|
|
|
 |
A.N.Khan,
and
P.N.Lewis
(2006).
Use of substrate analogs and mutagenesis to study substrate binding and catalysis in the Sir2 family of NAD-dependent protein deacetylases.
|
| |
J Biol Chem,
281,
11702-11711.
|
 |
|
|
|
|
 |
D.A.King,
B.E.Hall,
M.A.Iwamoto,
K.Z.Win,
J.F.Chang,
and
T.Ellenberger
(2006).
Domain structure and protein interactions of the silent information regulator Sir3 revealed by screening a nested deletion library of protein fragments.
|
| |
J Biol Chem,
281,
20107-20119.
|
 |
|
|
|
|
 |
K.G.Hoff,
J.L.Avalos,
K.Sens,
and
C.Wolberger
(2006).
Insights into the sirtuin mechanism from ternary complexes containing NAD+ and acetylated peptide.
|
| |
Structure,
14,
1231-1240.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Sheng
(2006).
A role of novel serpin maspin in tumor progression: the divergence revealed through efforts to converge.
|
| |
J Cell Physiol,
209,
631-635.
|
 |
|
|
|
|
 |
T.Huhtiniemi,
C.Wittekindt,
T.Laitinen,
J.Leppänen,
A.Salminen,
A.Poso,
and
M.Lahtela-Kakkonen
(2006).
Comparative and pharmacophore model for deacetylase SIRT1.
|
| |
J Comput Aided Mol Des,
20,
589-599.
|
 |
|
|
|
|
 |
D.C.Drummond,
C.O.Noble,
D.B.Kirpotin,
Z.Guo,
G.K.Scott,
and
C.C.Benz
(2005).
Clinical development of histone deacetylase inhibitors as anticancer agents.
|
| |
Annu Rev Pharmacol Toxicol,
45,
495-528.
|
 |
|
|
|
|
 |
E.A.Sickmier,
D.Brekasis,
S.Paranawithana,
J.B.Bonanno,
M.S.Paget,
S.K.Burley,
and
C.L.Kielkopf
(2005).
X-ray structure of a Rex-family repressor/NADH complex insights into the mechanism of redox sensing.
|
| |
Structure,
13,
43-54.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.Liszt,
E.Ford,
M.Kurtev,
and
L.Guarente
(2005).
Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase.
|
| |
J Biol Chem,
280,
21313-21320.
|
 |
|
|
|
|
 |
J.L.Avalos,
K.M.Bever,
and
C.Wolberger
(2005).
Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme.
|
| |
Mol Cell,
17,
855-868.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Biel,
V.Wascholowski,
and
A.Giannis
(2005).
Epigenetics--an epicenter of gene regulation: histones and histone-modifying enzymes.
|
| |
Angew Chem Int Ed Engl,
44,
3186-3216.
|
 |
|
|
|
|
 |
B.J.North,
and
E.Verdin
(2004).
Sirtuins: Sir2-related NAD-dependent protein deacetylases.
|
| |
Genome Biol,
5,
224.
|
 |
|
|
|
|
 |
G.Blander,
and
L.Guarente
(2004).
The Sir2 family of protein deacetylases.
|
| |
Annu Rev Biochem,
73,
417-435.
|
 |
|
|
|
|
 |
H.Park,
and
S.Lee
(2004).
Homology modeling, force field design, and free energy simulation studies to optimize the activities of histone deacetylase inhibitors.
|
| |
J Comput Aided Mol Des,
18,
375-388.
|
 |
|
|
|
|
 |
K.Zhao,
R.Harshaw,
X.Chai,
and
R.Marmorstein
(2004).
Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD(+)-dependent Sir2 histone/protein deacetylases.
|
| |
Proc Natl Acad Sci U S A,
101,
8563-8568.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.M.Iyer,
K.S.Makarova,
E.V.Koonin,
and
L.Aravind
(2004).
Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging.
|
| |
Nucleic Acids Res,
32,
5260-5279.
|
 |
|
|
|
|
 |
M.T.Schmidt,
B.C.Smith,
M.D.Jackson,
and
J.M.Denu
(2004).
Coenzyme specificity of Sir2 protein deacetylases: implications for physiological regulation.
|
| |
J Biol Chem,
279,
40122-40129.
|
 |
|
|
|
|
 |
J.M.Denu
(2003).
Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases.
|
| |
Trends Biochem Sci,
28,
41-48.
|
 |
|
|
|
|
 |
K.J.Bitterman,
O.Medvedik,
and
D.A.Sinclair
(2003).
Longevity regulation in Saccharomyces cerevisiae: linking metabolism, genome stability, and heterochromatin.
|
| |
Microbiol Mol Biol Rev,
67,
376.
|
 |
|
|
|
|
 |
K.Zhao,
X.Chai,
A.Clements,
and
R.Marmorstein
(2003).
Structure and autoregulation of the yeast Hst2 homolog of Sir2.
|
| |
Nat Struct Biol,
10,
864-871.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Zhao,
X.Chai,
and
R.Marmorstein
(2003).
Structure of the yeast Hst2 protein deacetylase in ternary complex with 2'-O-acetyl ADP ribose and histone peptide.
|
| |
Structure,
11,
1403-1411.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.D.Jackson,
M.T.Schmidt,
N.J.Oppenheimer,
and
J.M.Denu
(2003).
Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases.
|
| |
J Biol Chem,
278,
50985-50998.
|
 |
|
|
|
|
 |
M.Hirao,
J.Posakony,
M.Nelson,
H.Hruby,
M.Jung,
J.A.Simon,
and
A.Bedalov
(2003).
Identification of selective inhibitors of NAD+-dependent deacetylases using phenotypic screens in yeast.
|
| |
J Biol Chem,
278,
52773-52782.
|
 |
|
|
|
|
 |
S.C.Dryden,
F.A.Nahhas,
J.E.Nowak,
A.S.Goustin,
and
M.A.Tainsky
(2003).
Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle.
|
| |
Mol Cell Biol,
23,
3173-3185.
|
 |
|
|
|
|
 |
T.Senawong,
V.J.Peterson,
D.Avram,
D.M.Shepherd,
R.A.Frye,
S.Minucci,
and
M.Leid
(2003).
Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression.
|
| |
J Biol Chem,
278,
43041-43050.
|
 |
|
|
|
|
 |
D.M.Vigushin,
and
R.C.Coombes
(2002).
Histone deacetylase inhibitors in cancer treatment.
|
| |
Anticancer Drugs,
13,
1.
|
 |
|
|
|
|
 |
E.Langley,
M.Pearson,
M.Faretta,
U.M.Bauer,
R.A.Frye,
S.Minucci,
P.G.Pelicci,
and
T.Kouzarides
(2002).
Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence.
|
| |
EMBO J,
21,
2383-2396.
|
 |
|
|
|
|
 |
G.J.Hoppe,
J.C.Tanny,
A.D.Rudner,
S.A.Gerber,
S.Danaie,
S.P.Gygi,
and
D.Moazed
(2002).
Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation.
|
| |
Mol Cell Biol,
22,
4167-4180.
|
 |
|
|
|
|
 |
J.C.Tanny,
and
D.Moazed
(2002).
Recognition of acetylated proteins: lessons from an ancient family of enzymes.
|
| |
Structure,
10,
1290-1292.
|
 |
|
|
|
|
 |
J.H.Chang,
H.C.Kim,
K.Y.Hwang,
J.W.Lee,
S.P.Jackson,
S.D.Bell,
and
Y.Cho
(2002).
Structural basis for the NAD-dependent deacetylase mechanism of Sir2.
|
| |
J Biol Chem,
277,
34489-34498.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.T.Borra,
F.J.O'Neill,
M.D.Jackson,
B.Marshall,
E.Verdin,
K.R.Foltz,
and
J.M.Denu
(2002).
Conserved enzymatic production and biological effect of O-acetyl-ADP-ribose by silent information regulator 2-like NAD+-dependent deacetylases.
|
| |
J Biol Chem,
277,
12632-12641.
|
 |
|
|
|
|
 |
N.Ariel,
A.Zvi,
H.Grosfeld,
O.Gat,
Y.Inbar,
B.Velan,
S.Cohen,
and
A.Shafferman
(2002).
Search for potential vaccine candidate open reading frames in the Bacillus anthracis virulence plasmid pXO1: in silico and in vitro screening.
|
| |
Infect Immun,
70,
6817-6827.
|
 |
|
|
|
|
 |
R.Marmorstein
(2002).
Dehydrogenases, NAD, and transcription--what's the connection?
|
| |
Structure,
10,
1465-1466.
|
 |
|
|
|
|
 |
V.Kumar,
J.E.Carlson,
K.A.Ohgi,
T.A.Edwards,
D.W.Rose,
C.R.Escalante,
M.G.Rosenfeld,
and
A.K.Aggarwal
(2002).
Transcription corepressor CtBP is an NAD(+)-regulated dehydrogenase.
|
| |
Mol Cell,
10,
857-869.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Bedalov,
T.Gatbonton,
W.P.Irvine,
D.E.Gottschling,
and
J.A.Simon
(2001).
Identification of a small molecule inhibitor of Sir2p.
|
| |
Proc Natl Acad Sci U S A,
98,
15113-15118.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

