 |
PDBsum entry 1fb2

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
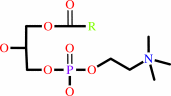 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Acta Crystallogr D Biol Crystallogr
57:1793-1798
(2001)
|
|
PubMed id:
|
|
|
|

|
| |
|
Regulation of catalytic function by molecular association: structure of phospholipase A2 from Daboia russelli pulchella (DPLA2) at 1.9 A resolution.
|
|
V.Chandra,
P.Kaur,
J.Jasti,
C.Betzel,
T.P.Singh.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of phospholipase A(2) from the venom of Daboia russelli
pulchella has been refined to an R factor of 0.216 using 17,922 reflections to
1.9 A resolution. The structure contains two crystallographically independent
molecules in the asymmetric unit. The overall conformations of the two molecules
are essentially the same except for three regions, namely the calcium-binding
loop including Trp31, the beta-wing and the C-terminal residues 119-131.
Although these differences have apparently been caused by molecular packing,
they seem to have functional relevance. Particularly noteworthy is the
conformation of Trp31, which is favourable for substrate binding in one molecule
as it is aligned with one of the side walls of the hydrophobic channel, whereas
in the other molecule it is located at the mouth of the channel, thereby
blocking the entry of substrates leading to loss of activity. This feature is
unique to the present structure and does not occur in the dimers and trimers of
other PLA(2)s.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1 A region of the final 2F[o] - F[c] electron-density map
contoured at 1.5  and
the corresponding refined model. The diagram was produced using
the program O (Jones et al., 1991[Jones, T. A., Zou, J., Cowan,
S. W. & Kjeldgaard, M. (1991). Acta Cryst. A47, 110-119.]). and
the corresponding refined model. The diagram was produced using
the program O (Jones et al., 1991[Jones, T. A., Zou, J., Cowan,
S. W. & Kjeldgaard, M. (1991). Acta Cryst. A47, 110-119.]).
|
 |
Figure 8.
Figure 8 Positioning of Trp31 vis-à-vis the hydrophobic binding
channel. The placement of Trp31 in molecule B (blue) reduces the
width of the channel, thus impairing its binding capability
while the corresponding width in molecule A (green) is optimum.
The distances between the two nearest atoms of Leu2 and Trp31
are 8.3 and 4.7 Å in molecules A and B, respectively.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the IUCr:
Acta Crystallogr D Biol Crystallogr
(2001,
57,
1793-1798)
copyright 2001.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.I.dos Santos,
M.Cintra-Francischinelli,
R.J.Borges,
C.A.Fernandes,
P.Pizzo,
A.C.Cintra,
A.S.Braz,
A.M.Soares,
and
M.R.Fontes
(2011).
Structural, functional, and bioinformatics studies reveal a new snake venom homologue phospholipase Aâ‚‚ class.
|
| |
Proteins,
79,
61-78.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.Faure,
V.T.Gowda,
and
R.C.Maroun
(2007).
Characterization of human coagulation factor Xa-binding site on Viperidae snake venom phospholipases A2 by affinity binding studies and molecular bioinformatics.
|
| |
BMC Struct Biol,
7,
82.
|
 |
|
|
|
|
 |
N.Singh,
T.Jabeen,
A.Pal,
S.Sharma,
M.Perbandt,
C.Betzel,
and
T.P.Singh
(2006).
Crystal structures of the complexes of a group IIA phospholipase A2 with two natural anti-inflammatory agents, anisic acid, and atropine reveal a similar mode of binding.
|
| |
Proteins,
64,
89.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Jabeen,
N.Singh,
R.K.Singh,
J.Jasti,
S.Sharma,
P.Kaur,
A.Srinivasan,
and
T.P.Singh
(2006).
Crystal structure of a heterodimer of phospholipase A2 from Naja naja sagittifera at 2.3 A resolution reveals the presence of a new PLA2-like protein with a novel cys 32-Cys 49 disulphide bridge with a bound sugar at the substrate-binding site.
|
| |
Proteins,
62,
329-337.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.Chandra,
J.Jasti,
P.Kaur,
S.Dey,
M.Perbandt,
A.Srinivasan,
C.h.Betzel,
and
T.P.Singh
(2002).
Crystal structure of a complex formed between a snake venom phospholipase A(2) and a potent peptide inhibitor Phe-Leu-Ser-Tyr-Lys at 1.8 A resolution.
|
| |
J Biol Chem,
277,
41079-41085.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

