 |
PDBsum entry 1f8r

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1f8r
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Crystal structure of l-amino acid oxidase from calloselasma rhodostoma complexed with citrate
|
|
Structure:
|
 |
L-amino acid oxidase. Chain: a, b, c, d. Ec: 1.4.3.2
|
|
Source:
|
 |
Calloselasma rhodostoma. Malayan pit viper. Organism_taxid: 8717. Secretion: venom
|
|
Biol. unit:
|
 |
 Dimer (from
)
Dimer (from
)
|
|
Resolution:
|
 |
|
2.00Å
|
R-factor:
|
0.185
|
R-free:
|
0.210
|
|
|
Authors:
|
 |
P.D.Pawelek,J.Cheah,R.Coulombe,P.Macheroux,S.Ghisla,A.Vrielink
|
Key ref:

|
 |
P.D.Pawelek
et al.
(2000).
The structure of L-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site.
EMBO J,
19,
4204-4215.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
04-Jul-00
|
Release date:
|
24-Aug-00
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P81382
(OXLA_CALRH) -
L-amino-acid oxidase from Calloselasma rhodostoma
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
516 a.a.
483 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.4.3.2
- L-amino-acid oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
an L-alpha-amino acid + O2 + H2O = a 2-oxocarboxylate + H2O2 + NH4+
|
 |
 |
 |
 |
 |
L-alpha-amino acid
|
+
|
 O2
O2
|
+
|
H2O
|
=
|
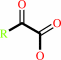 2-oxocarboxylate
2-oxocarboxylate
|
+
|
 H2O2
H2O2
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
EMBO J
19:4204-4215
(2000)
|
|
PubMed id:
|
|
|
|

|
| |
|
The structure of L-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site.
|
|
P.D.Pawelek,
J.Cheah,
R.Coulombe,
P.Macheroux,
S.Ghisla,
A.Vrielink.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The structure of L-amino acid oxidase (LAAO) from Calloselasma rhodostoma has
been determined to 2.0 A resolution in the presence of two ligands: citrate and
o-aminobenzoate (AB). The protomer consists of three domains: an FAD-binding
domain, a substrate-binding domain and a helical domain. The interface between
the substrate-binding and helical domains forms a 25 A long funnel, which
provides access to the active site. Three AB molecules are visible within the
funnel of the LAAO-AB complex; their orientations suggest the trajectory of the
substrate to the active site. The innermost AB molecule makes hydrogen bond
contacts with the active site residues, Arg90 and Gly464, and the aromatic
portion of the ligand is situated in a hydrophobic pocket. These contacts are
proposed to mimic those of the natural substrate. Comparison of LAAO with the
structure of mammalian D-amino acid oxidase reveals significant differences in
their modes of substrate entry. Furthermore, a mirror-symmetrical relationship
between the two substrate-binding sites is observed which facilitates
enantiomeric selectivity while preserving a common arrangement of the atoms
involved in catalysis.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2 Stereoview of the LAAO–citrate complex in the region
of (A) the FAD prosthetic group and (B) the citrate ligand. The
protein main chain is shown as a green coil, and specific amino
acid residues and the FAD molecule are depicted as
ball-and-stick models with yellow and gray bonds, respectively.
Water molecules are represented as red spheres. The hydrogen
bond contacts are shown as black dashed lines.
|
 |
Figure 4.
Figure 4 (A) Stereo view of the active sites of LAAO and porcine
DAAO (pcDAAO) with the respective FAD cofactors superimposed
along the isoalloxazine ring system. (B) Stereo view of the
active sites of LAAO and the mirror-image pcDAAO with the AB
ligands superimposed. Atoms and bonds are represented as capped
sticks, with those corresponding to DAAO being shown in green
and those from LAAO shown in red. The modeled His223 rotamer
optimally situated to abstract a proton from the amino group
from the substrate is shown in cyan. Superimposed AB molecules
are shown in magenta. The mirror plane co-incident with the
catalytic axis is shown in gray and those atoms involved in
catalysis that lie along the mirror plane are enlarged. (C)
Stereo representation of the modeled L-phenylalanine substrate
within the active site. Amino acid residues, the FAD and
L-phenylalanine molecules are shown in yellow, gray and cyan
bonds, respectively. Hydrogen bonds are depicted as black dashed
lines.
|
 |
|
|

|
| |
The above figures are
reprinted
from an Open Access publication published by Macmillan Publishers Ltd:
EMBO J
(2000,
19,
4204-4215)
copyright 2000.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.Georgieva,
M.Murakami,
M.Perband,
R.Arni,
and
C.Betzel
(2011).
The structure of a native l-amino acid oxidase, the major component of the Vipera ammodytes ammodytes venomic, reveals dynamic active site and quaternary structure stabilization by divalent ions.
|
| |
Mol Biosyst,
7,
379-384.
|
 |
|
|
|
|
 |
D.R.Rokyta,
K.P.Wray,
A.R.Lemmon,
E.M.Lemmon,
and
S.B.Caudle
(2011).
A high-throughput venom-gland transcriptome for the Eastern Diamondback Rattlesnake (Crotalus adamanteus) and evidence for pervasive positive selection across toxin classes.
|
| |
Toxicon,
57,
657-671.
|
 |
|
|
|
|
 |
F.Wang,
R.Li,
M.Xie,
and
A.Li
(2011).
The serum of rabbitfish (Siganus oramin) has antimicrobial activity to some pathogenic organisms and a novel serum L-amino acid oxidase is isolated.
|
| |
Fish Shellfish Immunol,
30,
1095-1108.
|
 |
|
|
|
|
 |
G.Kachalova,
K.Decker,
A.Holt,
and
H.D.Bartunik
(2011).
Crystallographic snapshots of the complete reaction cycle of nicotine degradation by an amine oxidase of the monoamine oxidase (MAO) family.
|
| |
Proc Natl Acad Sci U S A,
108,
4800-4805.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Sajevic,
A.Leonardi,
and
I.Križaj
(2011).
Haemostatically active proteins in snake venoms.
|
| |
Toxicon,
57,
627-645.
|
 |
|
|
|
|
 |
A.L.Hughes
(2010).
Origin and diversification of the L-amino oxidase family in innate immune defenses of animals.
|
| |
Immunogenetics,
62,
753-759.
|
 |
|
|
|
|
 |
M.S.Jorns,
Z.W.Chen,
and
F.S.Mathews
(2010).
Structural characterization of mutations at the oxygen activation site in monomeric sarcosine oxidase .
|
| |
Biochemistry,
49,
3631-3639.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.F.Fitzpatrick
(2010).
Oxidation of amines by flavoproteins.
|
| |
Arch Biochem Biophys,
493,
13-25.
|
 |
|
|
|
|
 |
F.Forneris,
E.Battaglioli,
A.Mattevi,
and
C.Binda
(2009).
New roles of flavoproteins in molecular cell biology: histone demethylase LSD1 and chromatin.
|
| |
FEBS J,
276,
4304-4312.
|
 |
|
|
|
|
 |
H.Gaweska,
M.Henderson Pozzi,
D.M.Schmidt,
D.G.McCafferty,
and
P.F.Fitzpatrick
(2009).
Use of pH and kinetic isotope effects to establish chemistry as rate-limiting in oxidation of a peptide substrate by LSD1.
|
| |
Biochemistry,
48,
5440-5445.
|
 |
|
|
|
|
 |
J.Arima,
C.Sasaki,
C.Sakaguchi,
H.Mizuno,
T.Tamura,
A.Kashima,
H.Kusakabe,
S.Sugio,
and
K.Inagaki
(2009).
Structural characterization of L-glutamate oxidase from Streptomyces sp. X-119-6.
|
| |
FEBS J,
276,
3894-3903.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.H.Pozzi,
V.Gawandi,
and
P.F.Fitzpatrick
(2009).
Mechanistic studies of para-substituted N,N'-dibenzyl-1,4-diaminobutanes as substrates for a mammalian polyamine oxidase.
|
| |
Biochemistry,
48,
12305-12313.
|
 |
|
|
|
|
 |
M.Henderson Pozzi,
V.Gawandi,
and
P.F.Fitzpatrick
(2009).
pH dependence of a mammalian polyamine oxidase: insights into substrate specificity and the role of lysine 315.
|
| |
Biochemistry,
48,
1508-1516.
|
 |
|
|
|
|
 |
P.Bhattacharya,
T.Ganeshan,
S.Nandi,
A.Srivastava,
P.Singh,
M.Rehan,
R.Rashkush,
N.Subbarao,
and
A.Lynn
(2009).
Analysis of oligomeric proteins during unfolding by pH and temperature.
|
| |
J Mol Model,
15,
1013-1025.
|
 |
|
|
|
|
 |
R.Doley,
and
R.M.Kini
(2009).
Protein complexes in snake venom.
|
| |
Cell Mol Life Sci,
66,
2851-2871.
|
 |
|
|
|
|
 |
S.Schriek,
U.Kahmann,
D.Staiger,
E.K.Pistorius,
and
K.P.Michel
(2009).
Detection of an L-amino acid dehydrogenase activity in Synechocystis sp. PCC 6803.
|
| |
J Exp Bot,
60,
1035-1046.
|
 |
|
|
|
|
 |
T.Senda,
M.Senda,
S.Kimura,
and
T.Ishida
(2009).
Redox control of protein conformation in flavoproteins.
|
| |
Antioxid Redox Signal,
11,
1741-1766.
|
 |
|
|
|
|
 |
D.Georgieva,
A.Kardas,
F.Buck,
M.Perbandt,
and
C.Betzel
(2008).
Isolation, crystallization and preliminary X-ray diffraction analysis of L-amino-acid oxidase from Vipera ammodytes ammodytes venom.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
64,
918-921.
|
 |
|
|
|
|
 |
G.Zhao,
R.C.Bruckner,
and
M.S.Jorns
(2008).
Identification of the oxygen activation site in monomeric sarcosine oxidase: role of Lys265 in catalysis.
|
| |
Biochemistry,
47,
9124-9135.
|
 |
|
|
|
|
 |
K.Ida,
M.Kurabayashi,
M.Suguro,
Y.Hiruma,
T.Hikima,
M.Yamomoto,
and
H.Suzuki
(2008).
Structural basis of proteolytic activation of L-phenylalanine oxidase from Pseudomonas sp. P-501.
|
| |
J Biol Chem,
283,
16584-16590.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Mandal,
and
D.Bhattacharyya
(2008).
Two L-amino acid oxidase isoenzymes from Russell's viper (Daboia russelli russelli) venom with different mechanisms of inhibition by substrate analogs.
|
| |
FEBS J,
275,
2078-2095.
|
 |
|
|
|
|
 |
S.R.Ande,
H.Fussi,
H.Knauer,
M.Murkovic,
S.Ghisla,
K.U.Fröhlich,
and
P.Macheroux
(2008).
Induction of apoptosis in yeast by L-amino acid oxidase from the Malayan pit viper Calloselasma rhodostoma.
|
| |
Yeast,
25,
349-357.
|
 |
|
|
|
|
 |
E.C.Ralph,
J.S.Hirschi,
M.A.Anderson,
W.W.Cleland,
D.A.Singleton,
and
P.F.Fitzpatrick
(2007).
Insights into the mechanism of flavoprotein-catalyzed amine oxidation from nitrogen isotope effects on the reaction of N-methyltryptophan oxidase.
|
| |
Biochemistry,
46,
7655-7664.
|
 |
|
|
|
|
 |
M.L.Boulland,
J.Marquet,
V.Molinier-Frenkel,
P.Möller,
C.Guiter,
F.Lasoudris,
C.Copie-Bergman,
M.Baia,
P.Gaulard,
K.Leroy,
and
F.Castellano
(2007).
Human IL4I1 is a secreted L-phenylalanine oxidase expressed by mature dendritic cells that inhibits T-lymphocyte proliferation.
|
| |
Blood,
110,
220-227.
|
 |
|
|
|
|
 |
P.F.Fitzpatrick
(2007).
Insights into the mechanisms of flavoprotein oxidases from kinetic isotope effects.
|
| |
J Labelled Comp Radiopharm,
50,
1016-1025.
|
 |
|
|
|
|
 |
E.C.Ralph,
M.A.Anderson,
W.W.Cleland,
and
P.F.Fitzpatrick
(2006).
Mechanistic studies of the flavoenzyme tryptophan 2-monooxygenase: deuterium and 15N kinetic isotope effects on alanine oxidation by an L-amino acid oxidase.
|
| |
Biochemistry,
45,
15844-15852.
|
 |
|
|
|
|
 |
I.M.Moustafa,
S.Foster,
A.Y.Lyubimov,
and
A.Vrielink
(2006).
Crystal structure of LAAO from Calloselasma rhodostoma with an L-phenylalanine substrate: insights into structure and mechanism.
|
| |
J Mol Biol,
364,
991.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.R.Ande,
P.R.Kommoju,
S.Draxl,
M.Murkovic,
P.Macheroux,
S.Ghisla,
and
E.Ferrando-May
(2006).
Mechanisms of cell death induction by L-amino acid oxidase, a major component of ophidian venom.
|
| |
Apoptosis,
11,
1439-1451.
|
 |
|
|
|
|
 |
A.Bazaa,
N.Marrakchi,
M.El Ayeb,
L.Sanz,
and
J.J.Calvete
(2005).
Snake venomics: comparative analysis of the venom proteomes of the Tunisian snakes Cerastes cerastes, Cerastes vipera and Macrovipera lebetina.
|
| |
Proteomics,
5,
4223-4235.
|
 |
|
|
|
|
 |
E.C.Ralph,
and
P.F.Fitzpatrick
(2005).
pH and kinetic isotope effects on sarcosine oxidation by N-methyltryptophan oxidase.
|
| |
Biochemistry,
44,
3074-3081.
|
 |
|
|
|
|
 |
Q.Lu,
J.M.Clemetson,
and
K.J.Clemetson
(2005).
Snake venoms and hemostasis.
|
| |
J Thromb Haemost,
3,
1791-1799.
|
 |
|
|
|
|
 |
T.Nishizawa,
C.C.Aldrich,
and
D.H.Sherman
(2005).
Molecular analysis of the rebeccamycin L-amino acid oxidase from Lechevalieria aerocolonigenes ATCC 39243.
|
| |
J Bacteriol,
187,
2084-2092.
|
 |
|
|
|
|
 |
H.Zhang,
M.Teng,
L.Niu,
Y.Wang,
Y.Wang,
Q.Liu,
Q.Huang,
Q.Hao,
Y.Dong,
and
P.Liu
(2004).
Purification, partial characterization, crystallization and structural determination of AHP-LAAO, a novel L-amino-acid oxidase with cell apoptosis-inducing activity from Agkistrodon halys pallas venom.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
974-977.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Koch,
C.Breithaupt,
R.Kiefersauer,
J.Freigang,
R.Huber,
and
A.Messerschmidt
(2004).
Crystal structure of protoporphyrinogen IX oxidase: a key enzyme in haem and chlorophyll biosynthesis.
|
| |
EMBO J,
23,
1720-1728.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Mörtl,
K.Diederichs,
W.Welte,
G.Molla,
L.Motteran,
G.Andriolo,
M.S.Pilone,
and
L.Pollegioni
(2004).
Structure-function correlation in glycine oxidase from Bacillus subtilis.
|
| |
J Biol Chem,
279,
29718-29727.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
P.Sobrado,
and
P.F.Fitzpatrick
(2003).
Analysis of the role of the active site residue Arg98 in the flavoprotein tryptophan 2-monooxygenase, a member of the L-amino oxidase family.
|
| |
Biochemistry,
42,
13826-13832.
|
 |
|
|
|
|
 |
P.Sobrado,
and
P.F.Fitzpatrick
(2003).
Identification of Tyr413 as an active site residue in the flavoprotein tryptophan 2-monooxygenase and analysis of its contribution to catalysis.
|
| |
Biochemistry,
42,
13833-13838.
|
 |
|
|
|
|
 |
T.Hamelryck
(2003).
Efficient identification of side-chain patterns using a multidimensional index tree.
|
| |
Proteins,
51,
96.
|
 |
|
|
|
|
 |
C.Binda,
A.Mattevi,
and
D.E.Edmondson
(2002).
Structure-function relationships in flavoenzyme-dependent amine oxidations: a comparison of polyamine oxidase and monoamine oxidase.
|
| |
J Biol Chem,
277,
23973-23976.
|
 |
|
|
|
|
 |
R.M.Geha,
K.Chen,
J.Wouters,
F.Ooms,
and
J.C.Shih
(2002).
Analysis of conserved active site residues in monoamine oxidase A and B and their three-dimensional molecular modeling.
|
| |
J Biol Chem,
277,
17209-17216.
|
 |
|
|
|
|
 |
W.T.Lowther,
H.Weissbach,
F.Etienne,
N.Brot,
and
B.W.Matthews
(2002).
The mirrored methionine sulfoxide reductases of Neisseria gonorrhoeae pilB.
|
| |
Nat Struct Biol,
9,
348-352.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Geyer,
T.B.Fitzpatrick,
P.D.Pawelek,
K.Kitzing,
A.Vrielink,
S.Ghisla,
and
P.Macheroux
(2001).
Structure and characterization of the glycan moiety of L-amino-acid oxidase from the Malayan pit viper Calloselasma rhodostoma.
|
| |
Eur J Biochem,
268,
4044-4053.
|
 |
|
|
|
|
 |
C.M.Harris,
L.Pollegioni,
and
S.Ghisla
(2001).
pH and kinetic isotope effects in d-amino acid oxidase catalysis.
|
| |
Eur J Biochem,
268,
5504-5520.
|
 |
|
|
|
|
 |
P.MacHeroux,
O.Seth,
C.Bollschweiler,
M.Schwarz,
M.Kurfürst,
L.C.Au,
and
S.Ghisla
(2001).
L-amino-acid oxidase from the Malayan pit viper Calloselasma rhodostoma. Comparative sequence analysis and characterization of active and inactive forms of the enzyme.
|
| |
Eur J Biochem,
268,
1679-1686.
|
 |
|
|
|
|
 |
S.Umhau,
L.Pollegioni,
G.Molla,
K.Diederichs,
W.Welte,
M.S.Pilone,
and
S.Ghisla
(2000).
The x-ray structure of D-amino acid oxidase at very high resolution identifies the chemical mechanism of flavin-dependent substrate dehydrogenation.
|
| |
Proc Natl Acad Sci U S A,
97,
12463-12468.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

