 |
PDBsum entry 1e3p

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Polyribonucleotide transferase
|
PDB id
|
|

|

|
1e3p
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.7.8
- polyribonucleotide nucleotidyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n+1) + phosphate = RNA(n) + a ribonucleoside 5'-diphosphate
|
 |
 |
 |
 |
 |
RNA(n+1)
|
+
|
 phosphate
phosphate
|
=
|
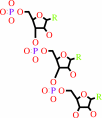 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure
8:1215-1226
(2000)
|
|
PubMed id:
|
|
|
|

|
| |
|
A duplicated fold is the structural basis for polynucleotide phosphorylase catalytic activity, processivity, and regulation.
|
|
M.F.Symmons,
G.H.Jones,
B.F.Luisi.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
BACKGROUND: Polynucleotide phosphorylase (PNPase) is a polyribonucleotide
nucleotidyl transferase (E.C.2.7.7.8) that degrades mRNA in prokaryotes.
Streptomyces antibioticus PNPase also assays as a guanosine 3'-diphosphate
5'-triphosphate (pppGpp) synthetase (E.C.2.7.6.5). It may function to coordinate
changes in mRNA lifetimes with pppGpp levels during the Streptomyces lifecycle.
RESULTS: The structure of S. antibioticus PNPase without bound RNA but with the
phosphate analog tungstate bound at the PNPase catalytic sites was determined by
X-ray crystallography and shows a trimeric multidomain protein with a central
channel. The structural core has a novel duplicated architecture formed by
association of two homologous domains. The tungstate derivative structure
reveals the PNPase active site in the second of these core domains.
Structure-based sequence analysis suggests that the pppGpp synthetase active
site is located in the first core domain. CONCLUSIONS: This is the first
structure of a PNPase and shows the structural basis for the trimer assembly,
the arrangement of accessory RNA binding domains, and the likely catalytic
residues of the PNPase active site. A possible function of the trimer channel is
as a contribution to both the processivity of degradation and the regulation of
PNPase action by RNA structural elements.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |
Figure 6.
Figure 6. View Into Putative PNPase Active Site(a)
Difference density at tungstate binding site with contours at 5
s (colored green). Overlaid is 2F[O]-F[C] sigmaa-weighted map
[45] and final refined model for tungstate derivative with
contours at 1.00 s (the model is colored red on outer and purple
on inner surfaces).(b) Secondary structure and key conserved
residues around the tungstate binding site. Sidechain atoms of
residues in tungstate binding loop and other key conserved
residues are shown. Residues of two additional conserved loops
are shown as colored Ca positions and numbered in corresponding
color. Residues 458-460 are shown with serine, asparagine, and
glycine colored pink, green, and black, respectively; residues
369-371 are shown with arginine, glycine, glutamic acid, and
threonine colored blue, black, orange, and pink, respectively;
and residues 412-413 are shown with glycine and glutamic acid as
black and orange, respectively

|
 |
|
|

|
| |
The above figure is
reprinted
by permission from Cell Press:
Structure
(2000,
8,
1215-1226)
copyright 2000.
|
|
| |
Figure was
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.K.Das,
S.K.Bhutia,
U.K.Sokhi,
R.Dash,
B.Azab,
D.Sarkar,
and
P.B.Fisher
(2011).
Human polynucleotide phosphorylase (hPNPase(old-35)): an evolutionary conserved gene with an expanding repertoire of RNA degradation functions.
|
| |
Oncogene,
30,
1733-1743.
|
 |
|
|
|
|
 |
Y.Peng,
Y.Luo,
T.Yu,
X.Xu,
K.Fan,
Y.Zhao,
and
K.Yang
(2011).
A Blue Native-PAGE analysis of membrane protein complexes in Clostridium thermocellum.
|
| |
BMC Microbiol,
11,
22.
|
 |
|
|
|
|
 |
B.Tsanova,
and
A.van Hoof
(2010).
Poring over exosome structure.
|
| |
EMBO Rep,
11,
900-901.
|
 |
|
|
|
|
 |
C.C.Yang,
Y.T.Wang,
Y.Y.Hsiao,
L.G.Doudeva,
P.H.Kuo,
S.Y.Chow,
and
H.S.Yuan
(2010).
Structural and biochemical characterization of CRN-5 and Rrp46: an exosome component participating in apoptotic DNA degradation.
|
| |
RNA,
16,
1748-1759.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.L.Ng,
D.G.Waterman,
A.A.Antson,
and
M.Ortiz-Lombardía
(2010).
Structure of the Methanothermobacter thermautotrophicus exosome RNase PH ring.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
522-528.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Lu,
F.Ding,
and
A.Ke
(2010).
Crystal structure of the S. solfataricus archaeal exosome reveals conformational flexibility in the RNA-binding ring.
|
| |
PLoS One,
5,
e8739.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.L.Gatewood,
and
G.H.Jones
(2010).
(p)ppGpp inhibits polynucleotide phosphorylase from streptomyces but not from Escherichia coli and increases the stability of bulk mRNA in Streptomyces coelicolor.
|
| |
J Bacteriol,
192,
4275-4280.
|
 |
|
|
|
|
 |
R.Tomecki,
K.Drazkowska,
and
A.Dziembowski
(2010).
Mechanisms of RNA degradation by the eukaryotic exosome.
|
| |
Chembiochem,
11,
938-945.
|
 |
|
|
|
|
 |
S.Andleeb,
I.Amin,
A.Bashir,
R.W.Briddon,
and
S.Mansoor
(2010).
Transient expression of βC1 protein differentially regulates host genes related to stress response, chloroplast and mitochondrial functions.
|
| |
Virol J,
7,
373.
|
 |
|
|
|
|
 |
A.E.Rawlings,
E.V.Blagova,
V.M.Levdikov,
M.J.Fogg,
K.S.Wilson,
and
A.J.Wilkinson
(2009).
The structure of Rph, an exoribonuclease from Bacillus anthracis, at 1.7 A resolution.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
65,
2-7.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Schneider,
E.Leung,
J.Brown,
and
D.Tollervey
(2009).
The N-terminal PIN domain of the exosome subunit Rrp44 harbors endonuclease activity and tethers Rrp44 to the yeast core exosome.
|
| |
Nucleic Acids Res,
37,
1127-1140.
|
 |
|
|
|
|
 |
D.Schaeffer,
B.Tsanova,
A.Barbas,
F.P.Reis,
E.G.Dastidar,
M.Sanchez-Rotunno,
C.M.Arraiano,
and
A.van Hoof
(2009).
The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities.
|
| |
Nat Struct Mol Biol,
16,
56-62.
|
 |
|
|
|
|
 |
F.Bonneau,
J.Basquin,
J.Ebert,
E.Lorentzen,
and
E.Conti
(2009).
The yeast exosome functions as a macromolecular cage to channel RNA substrates for degradation.
|
| |
Cell,
139,
547-559.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Nurmohamed,
B.Vaidialingam,
A.J.Callaghan,
and
B.F.Luisi
(2009).
Crystal structure of Escherichia coli polynucleotide phosphorylase core bound to RNase E, RNA and manganese: implications for catalytic mechanism and RNA degradosome assembly.
|
| |
J Mol Biol,
389,
17-33.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Lorentzen,
J.Basquin,
and
E.Conti
(2008).
Structural organization of the RNA-degrading exosome.
|
| |
Curr Opin Struct Biol,
18,
709-713.
|
 |
|
|
|
|
 |
J.C.Greimann,
and
C.D.Lima
(2008).
Reconstitution of RNA exosomes from human and Saccharomyces cerevisiae cloning, expression, purification, and activity assays.
|
| |
Methods Enzymol,
448,
185-210.
|
 |
|
|
|
|
 |
M.V.Falaleeva,
H.V.Chetverina,
V.I.Ugarov,
E.A.Uzlova,
and
A.B.Chetverin
(2008).
Factors influencing RNA degradation by Thermus thermophilus polynucleotide phosphorylase.
|
| |
FEBS J,
275,
2214-2226.
|
 |
|
|
|
|
 |
M.V.Navarro,
C.C.Oliveira,
N.I.Zanchin,
and
B.G.Guimarães
(2008).
Insights into the mechanism of progressive RNA degradation by the archaeal exosome.
|
| |
J Biol Chem,
283,
14120-14131.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Awano,
M.Inouye,
and
S.Phadtare
(2008).
RNase activity of polynucleotide phosphorylase is critical at low temperature in Escherichia coli and is complemented by RNase II.
|
| |
J Bacteriol,
190,
5924-5933.
|
 |
|
|
|
|
 |
S.A.Chang,
M.Cozad,
G.A.Mackie,
and
G.H.Jones
(2008).
Kinetics of polynucleotide phosphorylase: comparison of enzymes from Streptomyces and Escherichia coli and effects of nucleoside diphosphates.
|
| |
J Bacteriol,
190,
98.
|
 |
|
|
|
|
 |
S.L.Zimmer,
Z.Fei,
and
D.B.Stern
(2008).
Genome-based analysis of Chlamydomonas reinhardtii exoribonucleases and poly(A) polymerases predicts unexpected organellar and exosomal features.
|
| |
Genetics,
179,
125-136.
|
 |
|
|
|
|
 |
V.Portnoy,
G.Palnizky,
S.Yehudai-Resheff,
F.Glaser,
and
G.Schuster
(2008).
Analysis of the human polynucleotide phosphorylase (PNPase) reveals differences in RNA binding and response to phosphate compared to its bacterial and chloroplast counterparts.
|
| |
RNA,
14,
297-309.
|
 |
|
|
|
|
 |
Z.Shi,
W.Z.Yang,
S.Lin-Chao,
K.F.Chak,
and
H.S.Yuan
(2008).
Crystal structure of Escherichia coli PNPase: central channel residues are involved in processive RNA degradation.
|
| |
RNA,
14,
2361-2371.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Dziembowski,
E.Lorentzen,
E.Conti,
and
B.Séraphin
(2007).
A single subunit, Dis3, is essentially responsible for yeast exosome core activity.
|
| |
Nat Struct Mol Biol,
14,
15-22.
|
 |
|
|
|
|
 |
A.J.Carpousis
(2007).
The RNA degradosome of Escherichia coli: an mRNA-degrading machine assembled on RNase E.
|
| |
Annu Rev Microbiol,
61,
71-87.
|
 |
|
|
|
|
 |
E.Wahle
(2007).
Wrong PH for RNA degradation.
|
| |
Nat Struct Mol Biol,
14,
5-7.
|
 |
|
|
|
|
 |
H.W.Chen,
C.M.Koehler,
and
M.A.Teitell
(2007).
Human polynucleotide phosphorylase: location matters.
|
| |
Trends Cell Biol,
17,
600-608.
|
 |
|
|
|
|
 |
J.A.Rosenzweig,
B.Chromy,
A.Echeverry,
J.Yang,
B.Adkins,
G.V.Plano,
S.McCutchen-Maloney,
and
K.Schesser
(2007).
Polynucleotide phosphorylase independently controls virulence factor expression levels and export in Yersinia spp.
|
| |
FEMS Microbiol Lett,
270,
255-264.
|
 |
|
|
|
|
 |
J.A.Stead,
J.L.Costello,
M.J.Livingstone,
and
P.Mitchell
(2007).
The PMC2NT domain of the catalytic exosome subunit Rrp6p provides the interface for binding with its cofactor Rrp47p, a nucleic acid-binding protein.
|
| |
Nucleic Acids Res,
35,
5556-5567.
|
 |
|
|
|
|
 |
J.A.Worrall,
and
B.F.Luisi
(2007).
Information available at cut rates: structure and mechanism of ribonucleases.
|
| |
Curr Opin Struct Biol,
17,
128-137.
|
 |
|
|
|
|
 |
P.Marchi,
V.Longhi,
S.Zangrossi,
E.Gaetani,
F.Briani,
and
G.Dehò
(2007).
Autogenous regulation of Escherichia coli polynucleotide phosphorylase during cold acclimation by transcription termination and antitermination.
|
| |
Mol Genet Genomics,
278,
75-84.
|
 |
|
|
|
|
 |
S.Lin-Chao,
N.T.Chiou,
and
G.Schuster
(2007).
The PNPase, exosome and RNA helicases as the building components of evolutionarily-conserved RNA degradation machines.
|
| |
J Biomed Sci,
14,
523-532.
|
 |
|
|
|
|
 |
S.Vanacova,
and
R.Stefl
(2007).
The exosome and RNA quality control in the nucleus.
|
| |
EMBO Rep,
8,
651-657.
|
 |
|
|
|
|
 |
C.R.Ramos,
C.L.Oliveira,
I.L.Torriani,
and
C.C.Oliveira
(2006).
The Pyrococcus exosome complex: structural and functional characterization.
|
| |
J Biol Chem,
281,
6751-6759.
|
 |
|
|
|
|
 |
D.Sarkar,
and
P.B.Fisher
(2006).
Polynucleotide phosphorylase: an evolutionary conserved gene with an expanding repertoire of functions.
|
| |
Pharmacol Ther,
112,
243-263.
|
 |
|
|
|
|
 |
E.Lorentzen,
and
E.Conti
(2006).
The exosome and the proteasome: nano-compartments for degradation.
|
| |
Cell,
125,
651-654.
|
 |
|
|
|
|
 |
J.Mercante,
K.Suzuki,
X.Cheng,
P.Babitzke,
and
T.Romeo
(2006).
Comprehensive alanine-scanning mutagenesis of Escherichia coli CsrA defines two subdomains of critical functional importance.
|
| |
J Biol Chem,
281,
31832-31842.
|
 |
|
|
|
|
 |
K.Büttner,
K.Wenig,
and
K.P.Hopfner
(2006).
The exosome: a macromolecular cage for controlled RNA degradation.
|
| |
Mol Microbiol,
61,
1372-1379.
|
 |
|
|
|
|
 |
M.J.Marcaida,
M.A.DePristo,
V.Chandran,
A.J.Carpousis,
and
B.F.Luisi
(2006).
The RNA degradosome: life in the fast lane of adaptive molecular evolution.
|
| |
Trends Biochem Sci,
31,
359-365.
|
 |
|
|
|
|
 |
P.Walter,
F.Klein,
E.Lorentzen,
A.Ilchmann,
G.Klug,
and
E.Evguenieva-Hackenberg
(2006).
Characterization of native and reconstituted exosome complexes from the hyperthermophilic archaeon Sulfolobus solfataricus.
|
| |
Mol Microbiol,
62,
1076-1089.
|
 |
|
|
|
|
 |
Q.Liu,
J.C.Greimann,
and
C.D.Lima
(2006).
Reconstitution, activities, and structure of the eukaryotic RNA exosome.
|
| |
Cell,
127,
1223-1237.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.N.Rainey,
J.D.Glavin,
H.W.Chen,
S.W.French,
M.A.Teitell,
and
C.M.Koehler
(2006).
A new function in translocation for the mitochondrial i-AAA protease Yme1: import of polynucleotide phosphorylase into the intermembrane space.
|
| |
Mol Cell Biol,
26,
8488-8497.
|
 |
|
|
|
|
 |
S.E.Ygberg,
M.O.Clements,
A.Rytkönen,
A.Thompson,
D.W.Holden,
J.C.Hinton,
and
M.Rhen
(2006).
Polynucleotide phosphorylase negatively controls spv virulence gene expression in Salmonella enterica.
|
| |
Infect Immun,
74,
1243-1254.
|
 |
|
|
|
|
 |
Y.Zuo,
H.A.Vincent,
J.Zhang,
Y.Wang,
M.P.Deutscher,
and
A.Malhotra
(2006).
Structural basis for processivity and single-strand specificity of RNase II.
|
| |
Mol Cell,
24,
149-156.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.Sarkar,
E.S.Park,
L.Emdad,
A.Randolph,
K.Valerie,
and
P.B.Fisher
(2005).
Defining the domains of human polynucleotide phosphorylase (hPNPaseOLD-35) mediating cellular senescence.
|
| |
Mol Cell Biol,
25,
7333-7343.
|
 |
|
|
|
|
 |
E.Lorentzen,
and
E.Conti
(2005).
Structural basis of 3' end RNA recognition and exoribonucleolytic cleavage by an exosome RNase PH core.
|
| |
Mol Cell,
20,
473-481.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Lorentzen,
P.Walter,
S.Fribourg,
E.Evguenieva-Hackenberg,
G.Klug,
and
E.Conti
(2005).
The archaeal exosome core is a hexameric ring structure with three catalytic subunits.
|
| |
Nat Struct Mol Biol,
12,
575-581.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.J.Pruijn
(2005).
Doughnuts dealing with RNA.
|
| |
Nat Struct Mol Biol,
12,
562-564.
|
 |
|
|
|
|
 |
J.A.Rosenzweig,
G.Weltman,
G.V.Plano,
and
K.Schesser
(2005).
Modulation of yersinia type three secretion system by the S1 domain of polynucleotide phosphorylase.
|
| |
J Biol Chem,
280,
156-163.
|
 |
|
|
|
|
 |
K.Büttner,
K.Wenig,
and
K.P.Hopfner
(2005).
Structural framework for the mechanism of archaeal exosomes in RNA processing.
|
| |
Mol Cell,
20,
461-471.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Folichon,
F.Allemand,
P.Régnier,
and
E.Hajnsdorf
(2005).
Stimulation of poly(A) synthesis by Escherichia coli poly(A)polymerase I is correlated with Hfq binding to poly(A) tails.
|
| |
FEBS J,
272,
454-463.
|
 |
|
|
|
|
 |
U.Z.Littauer,
and
U.Z.Littauer
(2005).
From polynucleotide phosphorylase to neurobiology.
|
| |
J Biol Chem,
280,
38889-38897.
|
 |
|
|
|
|
 |
C.Venclovas,
K.Ginalski,
and
C.Kang
(2004).
Sequence-structure mapping errors in the PDB: OB-fold domains.
|
| |
Protein Sci,
13,
1594-1602.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.M.Choi,
E.Y.Park,
J.H.Kim,
S.K.Chang,
and
Y.Cho
(2004).
Probing the functional importance of the hexameric ring structure of RNase PH.
|
| |
J Biol Chem,
279,
755-764.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.S.Harlow,
A.Kadziola,
K.F.Jensen,
and
S.Larsen
(2004).
Crystal structure of the phosphorolytic exoribonuclease RNase PH from Bacillus subtilis and implications for its quaternary structure and tRNA binding.
|
| |
Protein Sci,
13,
668-677.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.E.Regonesi,
F.Briani,
A.Ghetta,
S.Zangrossi,
D.Ghisotti,
P.Tortora,
and
G.Dehò
(2004).
A mutation in polynucleotide phosphorylase from Escherichia coli impairing RNA binding and degradosome stability.
|
| |
Nucleic Acids Res,
32,
1006-1017.
|
 |
|
|
|
|
 |
P.Aloy,
B.Böttcher,
H.Ceulemans,
C.Leutwein,
C.Mellwig,
S.Fischer,
A.C.Gavin,
P.Bork,
G.Superti-Furga,
L.Serrano,
and
R.B.Russell
(2004).
Structure-based assembly of protein complexes in yeast.
|
| |
Science,
303,
2026-2029.
|
 |
|
|
|
|
 |
R.B.Russell,
F.Alber,
P.Aloy,
F.P.Davis,
D.Korkin,
M.Pichaud,
M.Topf,
and
A.Sali
(2004).
A structural perspective on protein-protein interactions.
|
| |
Curr Opin Struct Biol,
14,
313-324.
|
 |
|
|
|
|
 |
R.Parker,
and
H.Song
(2004).
The enzymes and control of eukaryotic mRNA turnover.
|
| |
Nat Struct Mol Biol,
11,
121-127.
|
 |
|
|
|
|
 |
S.C.Sinha,
B.N.Chaudhuri,
J.W.Burgner,
G.Yakovleva,
V.J.Davisson,
and
J.L.Smith
(2004).
Crystal structure of imidazole glycerol-phosphate dehydratase: duplication of an unusual fold.
|
| |
J Biol Chem,
279,
15491-15498.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.Ishii,
O.Nureki,
and
S.Yokoyama
(2003).
Crystal structure of the tRNA processing enzyme RNase PH from Aquifex aeolicus.
|
| |
J Biol Chem,
278,
32397-32404.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Rott,
G.Zipor,
V.Portnoy,
V.Liveanu,
and
G.Schuster
(2003).
RNA polyadenylation and degradation in cyanobacteria are similar to the chloroplast but different from Escherichia coli.
|
| |
J Biol Chem,
278,
15771-15777.
|
 |
|
|
|
|
 |
M.F.Symmons,
M.G.Williams,
B.F.Luisi,
G.H.Jones,
and
A.J.Carpousis
(2002).
Running rings around RNA: a superfamily of phosphate-dependent RNases.
|
| |
Trends Biochem Sci,
27,
11-18.
|
 |
|
|
|
|
 |
A.C.Jarrige,
N.Mathy,
and
C.Portier
(2001).
PNPase autocontrols its expression by degrading a double-stranded structure in the pnp mRNA leader.
|
| |
EMBO J,
20,
6845-6855.
|
 |
|
|
|
|
 |
S.Baginsky,
A.Shteiman-Kotler,
V.Liveanu,
S.Yehudai-Resheff,
M.Bellaoui,
R.E.Settlage,
J.Shabanowitz,
D.F.Hunt,
G.Schuster,
and
W.Gruissem
(2001).
Chloroplast PNPase exists as a homo-multimer enzyme complex that is distinct from the Escherichia coli degradosome.
|
| |
RNA,
7,
1464-1475.
|
 |
|
|
|
|
 |
S.Yehudai-Resheff,
M.Hirsh,
and
G.Schuster
(2001).
Polynucleotide phosphorylase functions as both an exonuclease and a poly(A) polymerase in spinach chloroplasts.
|
| |
Mol Cell Biol,
21,
5408-5416.
|
 |
|
|
|
|
 |
W.A.Breyer,
and
B.W.Matthews
(2001).
A structural basis for processivity.
|
| |
Protein Sci,
10,
1699-1711.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

