 |
PDBsum entry 1d0q

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.7.101
- Dna primase DnaG.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ssDNA + n NTP = ssDNA/pppN(pN)n-1 hybrid + (n-1) diphosphate
|
 |
 |
 |
 |
 |
ssDNA
|
+
|
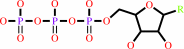 n
NTP
n
NTP
|
=
|
ssDNA/pppN(pN)n-1 hybrid
|
+
|
(n-1) diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure
8:231-239
(2000)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of the zinc-binding domain of Bacillus stearothermophilus DNA primase.
|
|
H.Pan,
D.B.Wigley.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
BACKGROUND: DNA primases catalyse the synthesis of the short RNA primers that
are required for DNA replication by DNA polymerases. Primases comprise three
functional domains: a zinc-binding domain that is responsible for template
recognition, a polymerase domain, and a domain that interacts with the
replicative helicase, DnaB. RESULTS: We present the crystal structure of the
zinc-binding domain of DNA primase from Bacillus stearothermophilus, determined
at 1.7 A resolution. This is the first high-resolution structural information
about any DNA primase. A model is discussed for the interaction of this domain
with the single-stranded DNA template. CONCLUSIONS: The structure of the DNA
primase zinc-binding domain confirms that the protein belongs to the zinc ribbon
subfamily. Structural comparison with other nucleic acid binding proteins
suggests that the beta sheet of primase is likely to be the DNA-binding surface,
with conserved residues on this surface being involved in the binding and
recognition of DNA.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |
Figure 4.
Figure 4. Structural comparison of members of the zinc
ribbon family. (a) TFIIB, (b) TFIIS, (c) RPB9 and (d) DNA
primase P12. The zinc ions are shown as a white ball.
|
 |
|
|

|
| |
The above figure is
reprinted
by permission from Cell Press:
Structure
(2000,
8,
231-239)
copyright 2000.
|
|
| |
Figure was
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.Li,
J.Liu,
L.Zhou,
H.Pei,
J.Zhou,
and
H.Xiang
(2010).
Two distantly homologous DnaG primases from Thermoanaerobacter tengcongensis exhibit distinct initiation specificities and priming activities.
|
| |
J Bacteriol,
192,
2670-2681.
|
 |
|
|
|
|
 |
K.Beck,
A.Vannini,
P.Cramer,
and
G.Lipps
(2010).
The archaeo-eukaryotic primase of plasmid pRN1 requires a helix bundle domain for faithful primer synthesis.
|
| |
Nucleic Acids Res,
38,
6707-6718.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.A.Larson,
M.A.Griep,
R.Bressani,
K.Chintakayala,
P.Soultanas,
and
S.H.Hinrichs
(2010).
Class-specific restrictions define primase interactions with DNA template and replicative helicase.
|
| |
Nucleic Acids Res,
38,
7167-7178.
|
 |
|
|
|
|
 |
K.Chintakayala,
M.A.Larson,
M.A.Griep,
S.H.Hinrichs,
and
P.Soultanas
(2008).
Conserved residues of the C-terminal p16 domain of primase are involved in modulating the activity of the bacterial primosome.
|
| |
Mol Microbiol,
68,
360-371.
|
 |
|
|
|
|
 |
J.E.Corn,
and
J.M.Berger
(2006).
Regulation of bacterial priming and daughter strand synthesis through helicase-primase interactions.
|
| |
Nucleic Acids Res,
34,
4082-4088.
|
 |
|
|
|
|
 |
J.Thirlway,
and
P.Soultanas
(2006).
In the Bacillus stearothermophilus DnaB-DnaG complex, the activities of the two proteins are modulated by distinct but overlapping networks of residues.
|
| |
J Bacteriol,
188,
1534-1539.
|
 |
|
|
|
|
 |
S.A.Koepsell,
M.A.Larson,
M.A.Griep,
and
S.H.Hinrichs
(2006).
Staphylococcus aureus helicase but not Escherichia coli helicase stimulates S. aureus primase activity and maintains initiation specificity.
|
| |
J Bacteriol,
188,
4673-4680.
|
 |
|
|
|
|
 |
X.C.Su,
P.M.Schaeffer,
K.V.Loscha,
P.H.Gan,
N.E.Dixon,
and
G.Otting
(2006).
Monomeric solution structure of the helicase-binding domain of Escherichia coli DnaG primase.
|
| |
FEBS J,
273,
4997-5009.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.J.Oakley,
K.V.Loscha,
P.M.Schaeffer,
E.Liepinsh,
G.Pintacuda,
M.C.Wilce,
G.Otting,
and
N.E.Dixon
(2005).
Crystal and solution structures of the helicase-binding domain of Escherichia coli primase.
|
| |
J Biol Chem,
280,
11495-11504.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.E.Corn,
P.J.Pease,
G.L.Hura,
and
J.M.Berger
(2005).
Crosstalk between primase subunits can act to regulate primer synthesis in trans.
|
| |
Mol Cell,
20,
391-401.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Syson,
J.Thirlway,
A.M.Hounslow,
P.Soultanas,
and
J.P.Waltho
(2005).
Solution structure of the helicase-interaction domain of the primase DnaG: a model for helicase activation.
|
| |
Structure,
13,
609-616.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.M.Iyer,
E.V.Koonin,
D.D.Leipe,
and
L.Aravind
(2005).
Origin and evolution of the archaeo-eukaryotic primase superfamily and related palm-domain proteins: structural insights and new members.
|
| |
Nucleic Acids Res,
33,
3875-3896.
|
 |
|
|
|
|
 |
P.Soultanas
(2005).
The bacterial helicase-primase interaction: a common structural/functional module.
|
| |
Structure,
13,
839-844.
|
 |
|
|
|
|
 |
S.H.Lao-Sirieix,
R.K.Nookala,
P.Roversi,
S.D.Bell,
and
L.Pellegrini
(2005).
Structure of the heterodimeric core primase.
|
| |
Nat Struct Mol Biol,
12,
1137-1144.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Thirlway,
I.J.Turner,
C.T.Gibson,
L.Gardiner,
K.Brady,
S.Allen,
C.J.Roberts,
and
P.Soultanas
(2004).
DnaG interacts with a linker region that joins the N- and C-domains of DnaB and induces the formation of 3-fold symmetric rings.
|
| |
Nucleic Acids Res,
32,
2977-2986.
|
 |
|
|
|
|
 |
M.Kato,
T.Ito,
G.Wagner,
C.C.Richardson,
and
T.Ellenberger
(2003).
Modular architecture of the bacteriophage T7 primase couples RNA primer synthesis to DNA synthesis.
|
| |
Mol Cell,
11,
1349-1360.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.N.Frick,
and
C.C.Richardson
(2001).
DNA primases.
|
| |
Annu Rev Biochem,
70,
39-80.
|
 |
|
|
|
|
 |
J.S.Stamler,
S.Lamas,
and
F.C.Fang
(2001).
Nitrosylation. the prototypic redox-based signaling mechanism.
|
| |
Cell,
106,
675-683.
|
 |
|
|
|
|
 |
J.L.Keck,
and
J.M.Berger
(2000).
DNA replication at high resolution.
|
| |
Chem Biol,
7,
R63-R71.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

