 |
PDBsum entry 1css

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxo-acid-lyase
|
PDB id
|
|

|

|
1css
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.3.3.1
- citrate (Si)-synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Citric acid cycle
|
 |
 |
 |
 |
 |
Reaction:
|
 |
oxaloacetate + acetyl-CoA + H2O = citrate + CoA + H+
|
 |
 |
 |
 |
 |
 oxaloacetate
oxaloacetate
|
+
|
 acetyl-CoA
acetyl-CoA
|
+
|
H2O
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
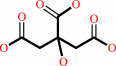 citrate
citrate
|
+
|
CoA
Bound ligand (Het Group name = )
matches with 88.68% similarity
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
34:15459-15466
(1995)
|
|
PubMed id:
|
|
|
|

|
| |
|
alpha-Fluoro acid and alpha-fluoro amide analogs of acetyl-CoA as inhibitors of citrate synthase: effect of pKa matching on binding affinity and hydrogen bond length.
|
|
B.Schwartz,
D.G.Drueckhammer,
K.C.Usher,
S.J.Remington.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
An alpha-fluoro acid analog and an alpha-fluoro amide analog of acetyl-CoA have
been synthesized. The ternary complexes of these inhibitors with oxaloacetate
and citrate synthase have been crystallized and their structures analyzed at 1.7
A resolution. The structures are similar to those reported for the corresponding
non-fluorinated analogs (Usher et al., 1994), with all forming unusually short
hydrogen bonds to Asp 375. The alpha-fluoro amide analog binds with an affinity
1.5-fold lower than that of a previously described amide analog lacking the
alpha-fluoro group. The alpha-fluoro acid analog binds with a 50-fold decreased
affinity relative to the corresponding unfluorinated analog. The binding
affinities are consistent with increased strengths of hydrogen bonds to Asp 375
with closer matching of pKa values between hydrogen bond donors and acceptors.
The results do not support any direct correlation between hydrogen bond strength
and hydrogen bond length in enzyme-inhibitor complexes.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Z.Gu,
D.G.Drueckhammer,
L.Kurz,
K.Liu,
D.P.Martin,
and
A.McDermott
(1999).
Solid state NMR studies of hydrogen bonding in a citrate synthase inhibitor complex.
|
| |
Biochemistry,
38,
8022-8031.
|
 |
|
|
|
|
 |
C.L.Perrin,
and
J.B.Nielson
(1997).
"Strong" hydrogen bonds in chemistry and biology.
|
| |
Annu Rev Phys Chem,
48,
511-544.
|
 |
|
|
|
|
 |
H.A.Charlier,
C.Narasimhan,
and
H.M.Miziorko
(1997).
Inactivation of 3-hydroxy-3-methylglutaryl-CoA synthase and other Acyl-CoA-utilizing enzymes by 3-Oxobutylsulfoxyl-CoA.
|
| |
Biochemistry,
36,
1551-1558.
|
 |
|
|
|
|
 |
L.C.Kurz,
J.H.Roble,
T.Nakra,
G.R.Drysdale,
J.M.Buzan,
B.Schwartz,
and
D.G.Drueckhammer
(1997).
Ability of single-site mutants of citrate synthase to catalyze proton transfer from the methyl group of dethiaacetyl-coenzyme A, a non-thioester substrate analog.
|
| |
Biochemistry,
36,
3981-3990.
|
 |
|
|
|
|
 |
C.T.Evans,
L.C.Kurz,
S.J.Remington,
and
P.A.Srere
(1996).
Active site mutants of pig citrate synthase: effects of mutations on the enzyme catalytic and structural properties.
|
| |
Biochemistry,
35,
10661-10672.
|
 |
|
|
|
|
 |
O.Hur,
C.Leja,
and
M.F.Dunn
(1996).
Evidence of a low-barrier hydrogen bond in the tryptophan synthase catalytic mechanism.
|
| |
Biochemistry,
35,
7378-7386.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

