 |
PDBsum entry 1bwc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1bwc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Structure of human glutathione reductase complexed with ajoene inhibitor and subversive substrate
|
|
Structure:
|
 |
Protein (glutathione reductase). Chain: a. Synonym: gr. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Cell: erythrocytes. Gene: gor. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Biol. unit:
|
 |
 Dimer (from PDB file)
Dimer (from PDB file)
|
|
Resolution:
|
 |
|
2.10Å
|
R-factor:
|
0.180
|
R-free:
|
0.230
|
|
|
Authors:
|
 |
H.Gallwitz,S.Bonse,A.Martinez-Cruz,I.Schlichting,K.Schumacher, R.L.Krauth-Siegel
|
|
Key ref:
|
 |
H.Gallwitz
et al.
(1999).
Ajoene is an inhibitor and subversive substrate of human glutathione reductase and Trypanosoma cruzi trypanothione reductase: crystallographic, kinetic, and spectroscopic studies.
J Med Chem,
42,
364-372.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
23-Sep-98
|
Release date:
|
20-Jul-99
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P00390
(GSHR_HUMAN) -
Glutathione reductase, mitochondrial from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
522 a.a.
461 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.8.1.7
- glutathione-disulfide reductase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 glutathione + NADP+ = glutathione disulfide + NADPH + H+
|
 |
 |
 |
 |
 |
 2
×
glutathione
2
×
glutathione
|
+
|
 NADP(+)
NADP(+)
|
=
|
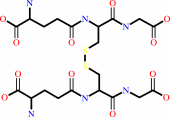 glutathione disulfide
glutathione disulfide
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
42:364-372
(1999)
|
|
PubMed id:
|
|
|
|

|
| |
|
Ajoene is an inhibitor and subversive substrate of human glutathione reductase and Trypanosoma cruzi trypanothione reductase: crystallographic, kinetic, and spectroscopic studies.
|
|
H.Gallwitz,
S.Bonse,
A.Martinez-Cruz,
I.Schlichting,
K.Schumacher,
R.L.Krauth-Siegel.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Ajoene ((E,Z)-4,5,9-trithiadodeca-1,6,11-triene 9-oxide), a garlic-derived
natural compound, is a covalent inhibitor as well as a substrate of human
glutathione reductase (GR) and Trypanosoma cruzi trypanothione reductase (TR).
The 2.1-A resolution crystal structure of GR inhibited by (E)-ajoene revealed a
mixed disulfide between the active site Cys58 and the CH2=CH-CH2-SO-CH2-CH=CH-S
moiety of ajoene. The modified enzyme has a markedly increased oxidase activity
when compared to free GR. GR reduces (Z)-ajoene with a kcat/Km of 6.8 x 10(3)
M-1 s-1 yielding 4,5,9-trithiadodeca-1, 6,11-triene (deoxyajoene) and
4,8,9,13-tetrathiahexadeca-1,6,10, 15-tetraene as stable reaction products. The
reaction leads also to the formation of single-electron reduced products and
concomitantly superoxide anion radicals as shown by coupling the reaction to the
reduction of cytochrome c. The interactions between the flavoenzymes and ajoene
are expected to increase the oxidative stress of the respective cell. The
antiparasitic and cytostatic actions of ajoene may at least in part be due to
the multiple effects on key enzymes of the antioxidant thiol metabolism.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
G.A.Holloway,
W.N.Charman,
A.H.Fairlamb,
R.Brun,
M.Kaiser,
E.Kostewicz,
P.M.Novello,
J.P.Parisot,
J.Richardson,
I.P.Street,
K.G.Watson,
and
J.B.Baell
(2009).
Trypanothione reductase high-throughput screening campaign identifies novel classes of inhibitors with antiparasitic activity.
|
| |
Antimicrob Agents Chemother,
53,
2824-2833.
|
 |
|
|
|
|
 |
S.Espinosa,
M.Solivan,
and
C.P.Vlaar
(2009).
Synthesis and redox-enzyme modulation by amino-1,4-dihydro-benzo[d][1,2]dithiine derivatives.
|
| |
Tetrahedron Lett,
50,
3023-3026.
|
 |
|
|
|
|
 |
T.Seefeldt,
Y.Zhao,
W.Chen,
A.S.Raza,
L.Carlson,
J.Herman,
A.Stoebner,
S.Hanson,
R.Foll,
and
X.Guan
(2009).
Characterization of a novel dithiocarbamate glutathione reductase inhibitor and its use as a tool to modulate intracellular glutathione.
|
| |
J Biol Chem,
284,
2729-2737.
|
 |
|
|
|
|
 |
U.Sharma,
T.Velpandian,
P.Sharma,
and
S.Singh
(2009).
Evaluation of anti-leishmanial activity of selected Indian plants known to have antimicrobial properties.
|
| |
Parasitol Res,
105,
1287-1293.
|
 |
|
|
|
|
 |
L.Thomaz,
R.Apitz-Castro,
A.F.Marques,
L.R.Travassos,
and
C.P.Taborda
(2008).
Experimental paracoccidioidomycosis: alternative therapy with ajoene, compound from Allium sativum, associated with sulfamethoxazole/trimethoprim.
|
| |
Med Mycol,
46,
113-118.
|
 |
|
|
|
|
 |
D.G.Covell,
A.Wallqvist,
R.Huang,
N.Thanki,
A.A.Rabow,
and
X.J.Lu
(2005).
Linking tumor cell cytotoxicity to mechanism of drug action: an integrated analysis of gene expression, small-molecule screening and structural databases.
|
| |
Proteins,
59,
403-433.
|
 |
|
|
|
|
 |
R.L.Krauth-Siegel,
H.Bauer,
and
R.H.Schirmer
(2005).
Dithiol proteins as guardians of the intracellular redox milieu in parasites: old and new drug targets in trypanosomes and malaria-causing plasmodia.
|
| |
Angew Chem Int Ed Engl,
44,
690-715.
|
 |
|
|
|
|
 |
W.Pearson,
H.J.Boermans,
W.J.Bettger,
B.W.McBride,
and
M.I.Lindinger
(2005).
Association of maximum voluntary dietary intake of freeze-dried garlic with Heinz body anemia in horses.
|
| |
Am J Vet Res,
66,
457-465.
|
 |
|
|
|
|
 |
B.Xu,
B.Monsarrat,
J.E.Gairin,
and
E.Girbal-Neuhauser
(2004).
Effect of ajoene, a natural antitumor small molecule, on human 20S proteasome activity in vitro and in human leukemic HL60 cells.
|
| |
Fundam Clin Pharmacol,
18,
171-180.
|
 |
|
|
|
|
 |
S.K.Banerjee,
P.K.Mukherjee,
and
S.K.Maulik
(2003).
Garlic as an antioxidant: the good, the bad and the ugly.
|
| |
Phytother Res,
17,
97.
|
 |
|
|
|
|
 |
S.K.Banerjee,
and
S.K.Maulik
(2002).
Effect of garlic on cardiovascular disorders: a review.
|
| |
Nutr J,
1,
4.
|
 |
|
|
|
|
 |
B.Hofmann,
H.Budde,
K.Bruns,
S.A.Guerrero,
H.M.Kalisz,
U.Menge,
M.Montemartini,
E.Nogoceke,
P.Steinert,
J.B.Wissing,
L.Flohé,
and
H.J.Hecht
(2001).
Structures of tryparedoxins revealing interaction with trypanothione.
|
| |
Biol Chem,
382,
459-471.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.Flohé,
H.J.Hecht,
and
P.Steinert
(1999).
Glutathione and trypanothione in parasitic hydroperoxide metabolism.
|
| |
Free Radic Biol Med,
27,
966-984.
|
 |
|
|
|
|
 |
R.L.Krauth-Siegel,
and
G.H.Coombs
(1999).
Enzymes of parasite thiol metabolism as drug targets.
|
| |
Parasitol Today,
15,
404-409.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

