 |
PDBsum entry 1arv

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Peroxidase (donor:h2o2 oxidoreductase)
|
PDB id
|
|

|

|
1arv
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.11.1.7
- peroxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 a phenolic donor + H2O2 = 2 a phenolic radical donor + 2 H2O
|
 |
 |
 |
 |
 |
2
×
a phenolic donor
|
+
|
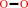 H2O2
H2O2
|
=
|
2
×
a phenolic radical donor
|
+
|
2
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Heme
|
 |
 |
 |
 |
 |
Heme
Bound ligand (Het Group name =
HEM)
matches with 95.45% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Biol Chem
270:21884-21892
(1995)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of cyanide- and triiodide-bound forms of Arthromyces ramosus peroxidase at different pH values. Perturbations of active site residues and their implication in enzyme catalysis.
|
|
K.Fukuyama,
N.Kunishima,
F.Amada,
T.Kubota,
H.Matsubara.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The structures of the cyanide and triiodide complexes of Arthromyces ramosus
peroxidase (ARP) at different pH values were investigated by x-ray
crystallography in order to examine the behavior of the invariant residues of
arginine (Arg-52) and distal histidine (His-56) during the enzyme reaction as
well as to provide the structural basis of the active site of peroxidase. The
models of the cyanide complexes at pH 7.5, 5.0, and 4.0, respectively, were
refined to the R-factors of 17.8, 17.8, and 18.5% using 7.0-1.6-A resolution
data, and those of the triiodide complexes at pH 6.5 and 5.0 refined to 16.9 and
16.8% using 7.0-1.9-A resolution data. The structures of the cyanide complexes
at pH 7.5, 5.0, and 4.0 are identical within experimental error. Cyanide ion
bound to the heme in the bent conformation rather than in the tilt conformation.
Upon cyanide ion binding, the N epsilon atom of His-56 moved toward the ion by
rotation of the imidazole ring around the C beta-C gamma bond, but there was
little conformational change in the remaining residues. The distance between the
N epsilon atom of His-56 and the nitrogen atom of the cyanide suggests the
presence of a hydrogen bond between them in the pH range investigated. In the
triiodide complexes, one of the two triiodides bound to ARP was located at the
distal side of the heme. When triiodide bound to ARP, unlike the rearrangement
of the distal arginine of cytochrome c peroxidase that occurs on formation of
the fluoride complex or compound I, the side chain of Arg-52 moved little. The
conformation of the side chain of His-56, however, changed markedly.
Conformational flexibility of His-56 appears to be a requisite for proton
translocation from one oxygen atom to the other of HOO- by acid-base catalysis
to produce compound I. The iron atom in each cyanide complex (low-spin ferric)
is located in the heme plane, whereas in each triiodide complex (high-spin
ferric) the iron atom is displaced from the plane about 0.2 A toward the
proximal side.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.H.Bryant,
M.Moll,
B.Y.Chen,
V.Y.Fofanov,
and
L.E.Kavraki
(2010).
Analysis of substructural variation in families of enzymatic proteins with applications to protein function prediction.
|
| |
BMC Bioinformatics,
11,
242.
|
 |
|
|
|
|
 |
J.Li,
B.C.Noll,
C.E.Schulz,
and
W.R.Scheidt
(2007).
New insights on the electronic and molecular structure of cyanide-ligated iron(III) porphyrinates.
|
| |
Inorg Chem,
46,
2286-2298.
|
 |
|
|
|
|
 |
K.Fukuyama,
and
T.Okada
(2007).
Structures of cyanide, nitric oxide and hydroxylamine complexes of Arthromyces ramosusperoxidase at 100 K refined to 1.3 A resolution: coordination geometries of the ligands to the haem iron.
|
| |
Acta Crystallogr D Biol Crystallogr,
63,
472-477.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.Huang,
and
P.R.Ortiz de Montellano
(2007).
Arthromyces ramosus peroxidase produces two chlorinating species.
|
| |
Biochem Biophys Res Commun,
355,
581-586.
|
 |
|
|
|
|
 |
G.Battistuzzi,
M.Bellei,
F.De Rienzo,
and
M.Sola
(2006).
Redox properties of the Fe3+/Fe2+ couple in Arthromyces ramosus class II peroxidase and its cyanide adduct.
|
| |
J Biol Inorg Chem,
11,
586-592.
|
 |
|
|
|
|
 |
H.Atamna,
and
K.Boyle
(2006).
Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer's disease.
|
| |
Proc Natl Acad Sci U S A,
103,
3381-3386.
|
 |
|
|
|
|
 |
J.M.Turner,
J.Graziano,
G.Spraggon,
and
P.G.Schultz
(2006).
Structural plasticity of an aminoacyl-tRNA synthetase active site.
|
| |
Proc Natl Acad Sci U S A,
103,
6483-6488.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
G.Battistuzzi,
M.Bellei,
M.Borsari,
G.Di Rocco,
A.Ranieri,
and
M.Sola
(2005).
Axial ligation and polypeptide matrix effects on the reduction potential of heme proteins probed on their cyanide adducts.
|
| |
J Biol Inorg Chem,
10,
643-651.
|
 |
|
|
|
|
 |
K.Houborg,
P.Harris,
J.Petersen,
P.Rowland,
J.C.Poulsen,
P.Schneider,
J.Vind,
and
S.Larsen
(2003).
Impact of the physical and chemical environment on the molecular structure of Coprinus cinereus peroxidase.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
989-996.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.Joosten,
C.Lokman,
C.A.Van Den Hondel,
and
P.J.Punt
(2003).
The production of antibody fragments and antibody fusion proteins by yeasts and filamentous fungi.
|
| |
Microb Cell Fact,
2,
1.
|
 |
|
|
|
|
 |
G.Evans,
and
G.Bricogne
(2002).
Triiodide derivatization and combinatorial counter-ion replacement: two methods for enhancing phasing signal using laboratory Cu Kalpha X-ray equipment.
|
| |
Acta Crystallogr D Biol Crystallogr,
58,
976-991.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Monzani,
G.Alzuet,
L.Casella,
C.Redaelli,
C.Bassani,
A.M.Sanangelantoni,
M.Gullotti,
L.de Gioia,
L.Santagostini,
and
F.Chillemi
(2000).
Properties and reactivity of myoglobin reconstituted with chemically modified protohemin complexes.
|
| |
Biochemistry,
39,
9571-9582.
|
 |
|
|
|
|
 |
M.Bolognesi,
C.Rosano,
R.Losso,
A.Borassi,
M.Rizzi,
J.B.Wittenberg,
A.Boffi,
and
P.Ascenzi
(1999).
Cyanide binding to Lucina pectinata hemoglobin I and to sperm whale myoglobin: an x-ray crystallographic study.
|
| |
Biophys J,
77,
1093-1099.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Henriksen,
D.J.Schuller,
K.Meno,
K.G.Welinder,
A.T.Smith,
and
M.Gajhede
(1998).
Structural interactions between horseradish peroxidase C and the substrate benzhydroxamic acid determined by X-ray crystallography.
|
| |
Biochemistry,
37,
8054-8060.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Sundaramoorthy,
J.Terner,
and
T.L.Poulos
(1998).
Stereochemistry of the chloroperoxidase active site: crystallographic and molecular-modeling studies.
|
| |
Chem Biol,
5,
461-473.
|
 |
|
|
|
|
 |
A.K.Abelskov,
A.T.Smith,
C.B.Rasmussen,
H.B.Dunford,
and
K.G.Welinder
(1997).
pH dependence and structural interpretation of the reactions of Coprinus cinereus peroxidase with hydrogen peroxide, ferulic acid, and 2,2'-azinobis.
|
| |
Biochemistry,
36,
9453-9463.
|
 |
|
|
|
|
 |
E.Balog,
K.Kis-Petik,
J.Fidy,
M.Köhler,
and
J.Friedrich
(1997).
Interpretation of multiple Q(0,0) bands in the absorption spectrum of Mg-mesoporphyrin embedded in horseradish peroxidase.
|
| |
Biophys J,
73,
397-405.
|
 |
|
|
|
|
 |
G.Nie,
and
S.D.Aust
(1997).
Spectral changes of lignin peroxidase during reversible inactivation.
|
| |
Biochemistry,
36,
5113-5119.
|
 |
|
|
|
|
 |
M.Gajhede,
D.J.Schuller,
A.Henriksen,
A.T.Smith,
and
T.L.Poulos
(1997).
Crystal structure of horseradish peroxidase C at 2.15 A resolution.
|
| |
Nat Struct Biol,
4,
1032-1038.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Tanaka,
K.Ishimori,
M.Mukai,
T.Kitagawa,
and
I.Morishima
(1997).
Catalytic activities and structural properties of horseradish peroxidase distal His42 --> Glu or Gln mutant.
|
| |
Biochemistry,
36,
9889-9898.
|
 |
|
|
|
|
 |
M.Tanaka,
S.Nagano,
K.Ishimori,
and
I.Morishima
(1997).
Hydrogen bond network in the distal site of peroxidases: spectroscopic properties of Asn70 --> Asp horseradish peroxidase mutant.
|
| |
Biochemistry,
36,
9791-9798.
|
 |
|
|
|
|
 |
J.W.Tams,
and
K.G.Welinder
(1996).
Unfolding and refolding of Coprinus cinereus peroxidase at high pH, in urea, and at high temperature. Effect of organic and ionic additives on these processes.
|
| |
Biochemistry,
35,
7573-7579.
|
 |
|
|
|
|
 |
N.C.Veitch,
Y.Gao,
and
K.G.Welinder
(1996).
The Asp245-->Asn mutant of Coprinus cinereus peroxidase. Characterization by 1H-NMR spectroscopy and comparison with the wild-type enzyme.
|
| |
Biochemistry,
35,
14370-14380.
|
 |
|
|
|
|
 |
S.Nagano,
M.Tanaka,
K.Ishimori,
Y.Watanabe,
and
I.Morishima
(1996).
Catalytic roles of the distal site asparagine-histidine couple in peroxidases.
|
| |
Biochemistry,
35,
14251-14258.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

