 |
PDBsum entry 1alg

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1alg
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.8.1.7
- glutathione-disulfide reductase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 glutathione + NADP+ = glutathione disulfide + NADPH + H+
|
 |
 |
 |
 |
 |
 2
×
glutathione
2
×
glutathione
|
+
|
 NADP(+)
NADP(+)
|
=
|
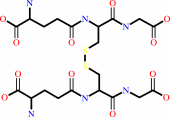 glutathione disulfide
glutathione disulfide
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
 FAD
FAD
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Eur J Biochem
245:273-282
(1997)
|
|
PubMed id:
|
|
|
|

|
| |
|
Denaturation and reactivation of dimeric human glutathione reductase--an assay for folding inhibitors.
|
|
A.Nordhoff,
C.Tziatzios,
J.A.van den Broek,
M.K.Schott,
H.R.Kalbitzer,
K.Becker,
D.Schubert,
R.H.Schirmer.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Human glutathione reductase (GR; which catalyzes the reaction NADPH + GSSG + H+
--> 2 GSH + NADP+) is an obligatory FAD-containing homodimer of known
geometry. Native human GR, a potential target of antimalarial and cytostatic
agents, cannot be dissociated by dilution or by means of subunit-interface
mimetics, similarly to well-studied viral dimeric proteins. However, ab initio
folding and/or dimerization of human GR can be inhibited by point mutations or
by peptides corresponding to subunit-interface areas, for example synthetic
peptide P11, which represents the intersubunit-contact helix H11. The structure
of this peptide, which might assist inhibitor design, was solved by
high-resolution NMR spectroscopy. Residues 440-453, were found to be alpha
helical in the isolated peptide. To quantitate the efficacy of inhibitors such
as P11, we developed the following unfolding/reactivation assay. The effects of
various guanidine hydrochloride (Gdn/HCl) concentrations were studied by
analytical ultracentrifugation. It was shown that human GR denatured by greater
than 3 M Gdn/HCl is monomeric and free of FAD. Circular-dichroism experiments at
223 nm indicated a half-life of approximately 20 s at 20 degrees C for the
unfolding process. To optimize the reactivation yield, four parameters [protein
concentration (x) in the range 0.3-10 microg/ml, cofactor supplementation,
temperature (y: 0-32 degrees C), and time (0-72 h)] were varied systematically,
and a reactivation score z was given to each constellation of parameters. This
type of analysis might be useful to optimize refolding and activation yields for
other proteins. For human GR, the highest recovery was found not to occur at one
of the corners of the x,y plane, but close to its center. Consequently, the
optimal assay conditions for folding and dimerization inhibitors are as follows.
The enzyme (at 300 microg/ml) is denatured by 5 M guanidine hydrochloride/5 mM
dithiothreitol, then reactivated by dilution to 1 microg/ml at pH 6.9 and 20
degrees C. In the absence of inhibitors, this procedure leads to 70% of the
control activity within 8 h. Peptides representing the upper subunit interface
(for instance residues 436-478) of human GR were found to inhibit refolding with
EC50% values in the micromolar range, whereas fragments from other regions of
the protein had no influence on this process. For peptide P11, the EC50% value
was 20 microM. In conclusion, hGR, enzyme with a tight intersubunit contact area
of 21 nm2, appears to be suitable for studying protein folding, dimerization,
and prosthetic-group complexation in the absence and presence of compounds that
inhibit these processes. There is a shortage, at least for oligomeric enzymes of
eukaryotes, of published systematic studies on protein (re)activation.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
B.Anselment,
D.Baerend,
E.Mey,
J.Buchner,
D.Weuster-Botz,
and
M.Haslbeck
(2010).
Experimental optimization of protein refolding with a genetic algorithm.
|
| |
Protein Sci,
19,
2085-2095.
|
 |
|
|
|
|
 |
A.C.Morais,
A.Chapeaurouge,
and
S.T.Ferreira
(2005).
Acid- and pressure-induced (un)folding of yeast glutathione reductase: competition between protein oligomerization and aggregation.
|
| |
Int J Biochem Cell Biol,
37,
1890-1899.
|
 |
|
|
|
|
 |
A.Loregian,
and
G.Palù
(2005).
Disruption of protein-protein interactions: towards new targets for chemotherapy.
|
| |
J Cell Physiol,
204,
750-762.
|
 |
|
|
|
|
 |
R.L.Krauth-Siegel,
H.Bauer,
and
R.H.Schirmer
(2005).
Dithiol proteins as guardians of the intracellular redox milieu in parasites: old and new drug targets in trypanosomes and malaria-causing plasmodia.
|
| |
Angew Chem Int Ed Engl,
44,
690-715.
|
 |
|
|
|
|
 |
K.Becker,
S.Rahlfs,
C.Nickel,
and
R.H.Schirmer
(2003).
Glutathione--functions and metabolism in the malarial parasite Plasmodium falciparum.
|
| |
Biol Chem,
384,
551-566.
|
 |
|
|
|
|
 |
P.R.Louzada,
A.Sebollela,
M.E.Scaramello,
and
S.T.Ferreira
(2003).
Predissociated dimers and molten globule monomers in the equilibrium unfolding of yeast glutathione reductase.
|
| |
Biophys J,
85,
3255-3261.
|
 |
|
|
|
|
 |
A.V.Veselovsky,
Y.D.Ivanov,
A.S.Ivanov,
A.I.Archakov,
P.Lewi,
and
P.Janssen
(2002).
Protein-protein interactions: mechanisms and modification by drugs.
|
| |
J Mol Recognit,
15,
405-422.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

