
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.4.2.1.20
- tryptophan synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Tryptophan Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate + L-serine = D-glyceraldehyde 3-phosphate + L-tryptophan + H2O
|
 |
 |
 |
 |
 |
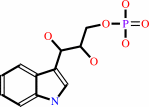 (1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate
(1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate
|
+
|
 L-serine
L-serine
|
=
|
 D-glyceraldehyde 3-phosphate
D-glyceraldehyde 3-phosphate
|
+
|
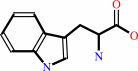 L-tryptophan
L-tryptophan
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
273:8553-8555
(1998)
|
|
PubMed id:
|
|
|
|

|
| |
|
Cryo-crystallography of a true substrate, indole-3-glycerol phosphate, bound to a mutant (alphaD60N) tryptophan synthase alpha2beta2 complex reveals the correct orientation of active site alphaGlu49.
|
|
S.Rhee,
E.W.Miles,
D.R.Davies.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The reversible cleavage of indole-3-glycerol by the alpha-subunit of tryptophan
synthase has been proposed to be catalyzed by alphaGlu49 and alphaAsp60.
Although previous x-ray crystallographic structures of the tryptophan synthase
alpha2beta2 complex showed an interaction between the carboxylate of alphaAsp60
and the bound inhibitor indole-3-propanol phosphate, the carboxylate of
alphaGlu49 was too distant to play its proposed role. To clarify the structural
and functional roles of alphaGlu49, we have determined crystal structures of a
mutant (alphaD60N) alpha2beta2 complex in the presence and absence of the true
substrate, indole-3-glycerol phosphate. The enzyme in the crystal cleaves
indole-3-glycerol phosphate very slowly at room temperature but not under
cryo-conditions of 95 K. The structure of the complex with the true substrate
obtained by cryo-crystallography reveals that indole-3-glycerol phosphate and
indole-3-propanol phosphate have similar binding modes but different torsion
angles. Most importantly, the side chain of alphaGlu49 interacts with 3-hydroxyl
group of indole-3-glycerol phosphate as proposed. The movement of the side chain
of alphaGlu49 into an extended conformation upon binding the true substrate
provides evidence for an induced fit mechanism. Our results demonstrate how
cryo-crystallography and mutagenesis can provide insight into enzyme mechanism.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |
Figure 1.
Fig. 1. A, the final 2F[o]  F[c] map
overlaid on the models of IGP and residues F[c] map
overlaid on the models of IGP and residues  Glu49 and Glu49 and
 Tyr175 in
the Tyr175 in
the  D60N-IGP
complex. The map was contoured at 0.6 D60N-IGP
complex. The map was contoured at 0.6  . B,
superposition between IGP (open circles) in the . B,
superposition between IGP (open circles) in the  D60N-IGP
and IPP (filled circles) in the D60N-IGP
and IPP (filled circles) in the  K87T-Ser-IPP
complex (7). The carboxylate of K87T-Ser-IPP
complex (7). The carboxylate of  Asp60 is
shown near the indole nitrogen of IPP and of IGP. Asp60 is
shown near the indole nitrogen of IPP and of IGP.
|
 |
|
|

|
| |
The above figure is
reprinted
by permission from the ASBMB:
J Biol Chem
(1998,
273,
8553-8555)
copyright 1998.
|
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.Nishio,
K.Ogasahara,
Y.Morimoto,
T.Tsukihara,
S.J.Lee,
and
K.Yutani
(2010).
Large conformational changes in the Escherichia coli tryptophan synthase beta(2) subunit upon pyridoxal 5'-phosphate binding.
|
| |
FEBS J,
277,
2157-2170.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.F.Dunn,
D.Niks,
H.Ngo,
T.R.Barends,
and
I.Schlichting
(2008).
Tryptophan synthase: the workings of a channeling nanomachine.
|
| |
Trends Biochem Sci,
33,
254-264.
|
 |
|
|
|
|
 |
T.R.Barends,
M.F.Dunn,
and
I.Schlichting
(2008).
Tryptophan synthase, an allosteric molecular factory.
|
| |
Curr Opin Chem Biol,
12,
593-600.
|
 |
|
|
|
|
 |
E.W.Miles
(2001).
Tryptophan synthase: a multienzyme complex with an intramolecular tunnel.
|
| |
Chem Rec,
1,
140-151.
|
 |
|
|
|
|
 |
E.Weber-Ban,
O.Hur,
C.Bagwell,
U.Banik,
L.H.Yang,
E.W.Miles,
and
M.F.Dunn
(2001).
Investigation of allosteric linkages in the regulation of tryptophan synthase: the roles of salt bridges and monovalent cations probed by site-directed mutation, optical spectroscopy, and kinetics.
|
| |
Biochemistry,
40,
3497-3511.
|
 |
|
|
|
|
 |
J.A.Gerlt,
and
P.C.Babbitt
(2001).
Divergent evolution of enzymatic function: mechanistically diverse superfamilies and functionally distinct suprafamilies.
|
| |
Annu Rev Biochem,
70,
209-246.
|
 |
|
|
|
|
 |
P.Rondard,
and
H.Bedouelle
(2000).
Mutational scanning of a hairpin loop in the tryptophan synthase beta-subunit implicated in allostery and substrate channeling.
|
| |
Biol Chem,
381,
1185-1193.
|
 |
|
|
|
|
 |
Y.X.Fan,
P.McPhie,
and
E.W.Miles
(2000).
Regulation of tryptophan synthase by temperature, monovalent cations, and an allosteric ligand. Evidence from Arrhenius plots, absorption spectra, and primary kinetic isotope effects.
|
| |
Biochemistry,
39,
4692-4703.
|
 |
|
|
|
|
 |
E.Woehl,
and
M.F.Dunn
(1999).
Mechanisms of monovalent cation action in enzyme catalysis: the first stage of the tryptophan synthase beta-reaction.
|
| |
Biochemistry,
38,
7118-7130.
|
 |
|
|
|
|
 |
I.Bahar,
and
R.L.Jernigan
(1999).
Cooperative fluctuations and subunit communication in tryptophan synthase.
|
| |
Biochemistry,
38,
3478-3490.
|
 |
|
|
|
|
 |
K.A.Denessiouk,
A.I.Denesyuk,
J.V.Lehtonen,
T.Korpela,
and
M.S.Johnson
(1999).
Common structural elements in the architecture of the cofactor-binding domains in unrelated families of pyridoxal phosphate-dependent enzymes.
|
| |
Proteins,
35,
250-261.
|
 |
|
|
|
|
 |
M.Weyand,
and
I.Schlichting
(1999).
Crystal structure of wild-type tryptophan synthase complexed with the natural substrate indole-3-glycerol phosphate.
|
| |
Biochemistry,
38,
16469-16480.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.L.Stoddard
(1998).
New results using Laue diffraction and time-resolved crystallography.
|
| |
Curr Opin Struct Biol,
8,
612-618.
|
 |
|
|
|
|
 |
J.N.Jansonius
(1998).
Structure, evolution and action of vitamin B6-dependent enzymes.
|
| |
Curr Opin Struct Biol,
8,
759-769.
|
 |
|
|
|
|
 |
S.Rhee,
E.W.Miles,
A.Mozzarelli,
and
D.R.Davies
(1998).
Cryocrystallography and microspectrophotometry of a mutant (alpha D60N) tryptophan synthase alpha 2 beta 2 complex reveals allosteric roles of alpha Asp60.
|
| |
Biochemistry,
37,
10653-10659.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

