 |
PDBsum entry 1a2a

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Presynaptic neurotoxin
|
PDB id
|
|

|

|
1a2a
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Presynaptic neurotoxin
|
 |
|
Title:
|
 |
Agkistrotoxin, a phospholipase a2-type presynaptic neurotoxin from agkistrodon halys pallas
|
|
Structure:
|
 |
Phospholipase a2. Chain: a, b, c, d, e, f, g, h. Synonym: agkistrotoxin, atx. Ec: 3.1.1.4
|
|
Source:
|
 |
Gloydius halys. Halys viper. Organism_taxid: 8714
|
|
Biol. unit:
|
 |
 Tetramer (from
)
Tetramer (from
)
|
|
Resolution:
|
 |
|
2.80Å
|
R-factor:
|
0.207
|
R-free:
|
0.285
|
|
|
Authors:
|
 |
L.Tang,Y.Zhou,Z.Lin
|
Key ref:

|
 |
L.Tang
et al.
(1998).
Crystal structure of agkistrodotoxin, a phospholipase A2-type presynaptic neurotoxin from agkistrodon halys pallas.
J Mol Biol,
282,
1.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
25-Dec-97
|
Release date:
|
13-Jan-99
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P14421
(PA2N_GLOHA) -
Neutral phospholipase A2 agkistrodotoxin from Gloydius halys
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
122 a.a.
122 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
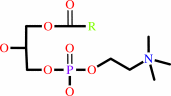 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
282:1
(1998)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of agkistrodotoxin, a phospholipase A2-type presynaptic neurotoxin from agkistrodon halys pallas.
|
|
L.Tang,
Y.C.Zhou,
Z.J.Lin.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of agkistrodotoxin containing eight copies of molecules in
the asymmetric unit has been determined at 2.8 A resolution to a
crystallographic R factor of 0.207 by the molecular replacement technique. Two
spatially adjacent regions of agkistrodotoxin molecule, turn 55-61 and stretch
85-91, are remarkably different from those of non-neurotoxic isoforms in
conformation and electrostatic characteristics. These regions are likely to be
involved in the recognition of agkistrodotoxin towards the specific receptor at
the presynaptic membrane. The structural comparison of the interfacial
recognition site with non-neurotoxic isoforms reveals a decreased hydrophobicity
and lack of residues with bulky hydrophobic side-chains (i.e. Trp) to serve as
membrane anchors. This structural feature of agkistrodotoxin may be related to
the reduced non-specific binding of the toxin to non-targeted membrane before it
arrives at the presynaptic membrane and recognizes the putative receptor. A
unique hydrophobic patch including residues I19, P20, F21, A23, F24, M118 and
F119 is found on the surface of the molecule near the entrance of the
hydrophobic channel which plays an important role in crystal packing. The
interaction mode between the patches might give a clue to the binding of the
neurotoxin on the membrane. The agkistrodotoxin molecules in the asymmetric unit
form two tetramers and each tetramer exhibits a novel "dimer of
dimers"-like structure. A molecule-spanning four-stranded antiparallel
beta-sheet is formed by the beta-wings of two molecules within a tetramer.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2. (a) Stereo view of the local structure of turn
55-61 and stretch 85-91. H-bonds are shown as broken lines. (b)
2F[o] -F[c] electron density map contoured at 1.3s of the
proposed neurotoxic site.
|
 |
Figure 3.
Figure 3. Stereo view of contacted hydrophobic patches from
molecule A of a tetramer (thick line) and molecule D of a
neighboring tetramer (thin line). The side-chains of residues
I19, P20, F21, A23, F24, M118 and F119 are shown.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(1998,
282,
1-0)
copyright 1998.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
P.H.Kao,
K.C.Chen,
S.R.Lin,
and
L.S.Chang
(2008).
The structural and functional contribution of N-terminal region and His-47 on Taiwan cobra phospholipase A2.
|
| |
J Pept Sci,
14,
342-348.
|
 |
|
|
|
|
 |
G.Faure,
V.T.Gowda,
and
R.C.Maroun
(2007).
Characterization of human coagulation factor Xa-binding site on Viperidae snake venom phospholipases A2 by affinity binding studies and molecular bioinformatics.
|
| |
BMC Struct Biol,
7,
82.
|
 |
|
|
|
|
 |
N.Singh,
T.Jabeen,
A.Pal,
S.Sharma,
M.Perbandt,
C.Betzel,
and
T.P.Singh
(2006).
Crystal structures of the complexes of a group IIA phospholipase A2 with two natural anti-inflammatory agents, anisic acid, and atropine reveal a similar mode of binding.
|
| |
Proteins,
64,
89.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Jabeen,
N.Singh,
R.K.Singh,
J.Jasti,
S.Sharma,
P.Kaur,
A.Srinivasan,
and
T.P.Singh
(2006).
Crystal structure of a heterodimer of phospholipase A2 from Naja naja sagittifera at 2.3 A resolution reveals the presence of a new PLA2-like protein with a novel cys 32-Cys 49 disulphide bridge with a bound sugar at the substrate-binding site.
|
| |
Proteins,
62,
329-337.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.Gao,
V.G.Starkov,
V.I.Tsetlin,
Y.N.Utkin,
Z.J.Lin,
and
R.C.Bi
(2005).
Isolation and preliminary crystallographic studies of two new phospholipases A2 from Vipera nikolskii venom.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
61,
189-192.
|
 |
|
|
|
|
 |
Y.Yamazaki,
H.Koike,
Y.Sugiyama,
K.Motoyoshi,
T.Wada,
S.Hishinuma,
M.Mita,
and
T.Morita
(2002).
Cloning and characterization of novel snake venom proteins that block smooth muscle contraction.
|
| |
Eur J Biochem,
269,
2708-2715.
|
 |
|
|
|
|
 |
S.Banumathi,
K.R.Rajashankar,
C.Nötzel,
B.Aleksiev,
T.P.Singh,
N.Genov,
and
C.Betzel
(2001).
Structure of the neurotoxic complex vipoxin at 1.4 A resolution.
|
| |
Acta Crystallogr D Biol Crystallogr,
57,
1552-1559.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.H.Lee,
M.T.da Silva Giotto,
S.Marangoni,
M.H.Toyama,
I.Polikarpov,
and
R.C.Garratt
(2001).
Structural basis for low catalytic activity in Lys49 phospholipases A2--a hypothesis: the crystal structure of piratoxin II complexed to fatty acid.
|
| |
Biochemistry,
40,
28-36.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Zhao,
S.Song,
Z.Lin,
and
Y.Zhou
(1999).
Refined structure of basic phospholipase A2 from venom ofAgkistrodon halys Pallas in orthorhombic crystal form I at 0.25 nm resolution.
|
| |
Sci China C Life Sci,
42,
80-89.
|
 |
|
|
|
|
 |
L.Tang,
Y.C.Zhou,
and
Z.J.Lin
(1999).
Structure of agkistrodotoxin in an orthorhombic crystal form with six molecules per asymmetric unit.
|
| |
Acta Crystallogr D Biol Crystallogr,
55,
1986-1996.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

