 |
PDBsum entry 1ypi

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Isomerase(intramolecular oxidoreductase)
|
PDB id
|
|

|

|
1ypi
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.5.3.1.1
- triose-phosphate isomerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
D-glyceraldehyde 3-phosphate = dihydroxyacetone phosphate
|
 |
 |
 |
 |
 |
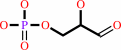 D-glyceraldehyde 3-phosphate
D-glyceraldehyde 3-phosphate
|
=
|
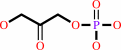 dihydroxyacetone phosphate
dihydroxyacetone phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
29:6609-6618
(1990)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of yeast triosephosphate isomerase at 1.9-A resolution.
|
|
E.Lolis,
T.Alber,
R.C.Davenport,
D.Rose,
F.C.Hartman,
G.A.Petsko.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The structure of yeast triosephosphate isomerase (TIM) has been solved at 3.0-A
resolution and refined at 1.9-A resolution to an R factor of 21.0%. The final
model consists of all non-hydrogen atoms in the polypeptide chain and 119 water
molecules, a number of which are found in the interior of the protein. The
structure of the active site clearly indicates that the carboxylate of the
catalytic base, Glu 165, is involved in a hydrogen-bonding interaction with the
hydroxyl of Ser 96. In addition, the interactions of the other active site
residues, Lys 12 and His 95, are also discussed. For the first time in any TIM
structure, the "flexible loop" has well-defined density; the conformation of the
loop in this structure is stabilized by a crystal contact. Analysis of the
subunit interface of this dimeric enzyme hints at the source of the specificity
of one subunit for another and allows us to estimate an association constant of
10(14)-10(16) M-1 for the two monomers. The analysis also suggests that the
interface may be a particularly good target for drug design. The conserved
positions (20%) among sequences from 13 sources ranging on the evolutionary
scale from Escherichia coli to humans reveal the intense pressure to maintain
the active site structure.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.Samanta,
M.Banerjee,
M.R.Murthy,
H.Balaram,
and
P.Balaram
(2011).
Probing the role of the fully conserved Cys126 in triosephosphate isomerase by site-specific mutagenesis - distal effects on dimer stability.
|
| |
FEBS J,
278,
1932-1943.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.J.Nolan,
A.R.Jex,
A.Pangasa,
N.D.Young,
A.J.Campbell,
M.Stevens,
and
R.B.Gasser
(2010).
Analysis of nucleotide variation within the triose-phosphate isomerase gene of Giardia duodenalis from sheep and its zoonotic implications.
|
| |
Electrophoresis,
31,
287-298.
|
 |
|
|
|
|
 |
X.Zhang,
Y.B.Teng,
J.P.Liu,
Y.X.He,
K.Zhou,
Y.Chen,
and
C.Z.Zhou
(2010).
Structural insights into the catalytic mechanism of the yeast pyridoxal 5-phosphate synthase Snz1.
|
| |
Biochem J,
432,
445-450.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.J.Juszczak,
and
R.Z.Desamero
(2009).
Extension of the tryptophan chi2,1 dihedral angle-W3 band frequency relationship to a full rotation: correlations and caveats.
|
| |
Biochemistry,
48,
2777-2787.
|
 |
|
|
|
|
 |
S.Mukherjee,
D.Dutta,
B.Saha,
and
A.K.Das
(2009).
Expression, purification, crystallization and preliminary X-ray diffraction studies of triosephosphate isomerase from methicillin-resistant Staphylococcus aureus (MRSA252).
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
65,
398-401.
|
 |
|
|
|
|
 |
S.R.Devenish,
and
J.A.Gerrard
(2009).
The role of quaternary structure in (beta/alpha)(8)-barrel proteins: evolutionary happenstance or a higher level of structure-function relationships?
|
| |
Org Biomol Chem,
7,
833-839.
|
 |
|
|
|
|
 |
S.S.Thakur,
P.D.Deepalakshmi,
P.Gayathri,
M.Banerjee,
M.R.Murthy,
and
P.Balaram
(2009).
Detection of the protein dimers, multiple monomeric states and hydrated forms of Plasmodium falciparum triosephosphate isomerase in the gas phase.
|
| |
Protein Eng Des Sel,
22,
289-304.
|
 |
|
|
|
|
 |
Y.Wang,
R.B.Berlow,
and
J.P.Loria
(2009).
Role of loop-loop interactions in coordinating motions and enzymatic function in triosephosphate isomerase.
|
| |
Biochemistry,
48,
4548-4556.
|
 |
|
|
|
|
 |
A.C.O'Donoghue,
T.L.Amyes,
and
J.P.Richard
(2008).
Slow proton transfer from the hydrogen-labelled carboxylic acid side chain (Glu-165) of triosephosphate isomerase to imidazole buffer in D(2)O.
|
| |
Org Biomol Chem,
6,
391-396.
|
 |
|
|
|
|
 |
C.A.Smith,
and
T.Kortemme
(2008).
Backrub-like backbone simulation recapitulates natural protein conformational variability and improves mutant side-chain prediction.
|
| |
J Mol Biol,
380,
742-756.
|
 |
|
|
|
|
 |
C.H.Chu,
Y.J.Lai,
H.Huang,
and
Y.J.Sun
(2008).
Kinetic and structural properties of triosephosphate isomerase from Helicobacter pylori.
|
| |
Proteins,
71,
396-406.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Gulotta,
L.Qiu,
R.Desamero,
J.Rösgen,
D.W.Bolen,
and
R.Callender
(2007).
Effects of cell volume regulating osmolytes on glycerol 3-phosphate binding to triosephosphate isomerase.
|
| |
Biochemistry,
46,
10055-10062.
|
 |
|
|
|
|
 |
T.L.Amyes,
and
J.P.Richard
(2007).
Enzymatic catalysis of proton transfer at carbon: activation of triosephosphate isomerase by phosphite dianion.
|
| |
Biochemistry,
46,
5841-5854.
|
 |
|
|
|
|
 |
M.G.Botelho,
A.W.Rietveld,
and
S.T.Ferreira
(2006).
Long-lived conformational isomerism of protein dimers: the role of the free energy of subunit association.
|
| |
Biophys J,
91,
2826-2832.
|
 |
|
|
|
|
 |
A.Shukla,
and
P.Guptasarma
(2004).
Folding of beta/alpha-unit scrambled forms of S. cerevisiae triosephosphate isomerase: Evidence for autonomy of substructure formation and plasticity of hydrophobic and hydrogen bonding interactions in core of (beta/alpha)8-barrel.
|
| |
Proteins,
55,
548-557.
|
 |
|
|
|
|
 |
P.K.Agarwal
(2004).
Cis/trans isomerization in HIV-1 capsid protein catalyzed by cyclophilin A: insights from computational and theoretical studies.
|
| |
Proteins,
56,
449-463.
|
 |
|
|
|
|
 |
G.Alagona,
C.Ghio,
and
P.A.Kollman
(2003).
The intramolecular mechanism for the second proton transfer in triosephosphate isomerase (TIM): a QM/FE approach.
|
| |
J Comput Chem,
24,
46-56.
|
 |
|
|
|
|
 |
G.Jogl,
S.Rozovsky,
A.E.McDermott,
and
L.Tong
(2003).
Optimal alignment for enzymatic proton transfer: structure of the Michaelis complex of triosephosphate isomerase at 1.2-A resolution.
|
| |
Proc Natl Acad Sci U S A,
100,
50-55.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Parthasarathy,
K.Eaazhisai,
H.Balaram,
P.Balaram,
and
M.R.Murthy
(2003).
Structure of Plasmodium falciparum triose-phosphate isomerase-2-phosphoglycerate complex at 1.1-A resolution.
|
| |
J Biol Chem,
278,
52461-52470.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.H.Moreau,
A.W.Rietveld,
and
S.T.Ferreira
(2003).
Persistent conformational heterogeneity of triosephosphate isomerase: separation and characterization of conformational isomers in solution.
|
| |
Biochemistry,
42,
14831-14837.
|
 |
|
|
|
|
 |
H.Reyes-Vivas,
E.Martínez-Martínez,
G.Mendoza-Hernández,
G.López-Velázquez,
R.Pérez-Montfort,
M.Tuena de Gómez-Puyou,
and
A.Gómez-Puyou
(2002).
Susceptibility to proteolysis of triosephosphate isomerase from two pathogenic parasites: characterization of an enzyme with an intact and a nicked monomer.
|
| |
Proteins,
48,
580-590.
|
 |
|
|
|
|
 |
A.Merico,
F.Rodrigues,
M.Côrte-Real,
D.Porro,
B.M.Ranzi,
and
C.Compagno
(2001).
Isolation and sequence analysis of the gene encoding triose phosphate isomerase from Zygosaccharomyces bailii.
|
| |
Yeast,
18,
775-780.
|
 |
|
|
|
|
 |
F.Joubert,
A.W.Neitz,
and
A.I.Louw
(2001).
Structure-based inhibitor screening: a family of sulfonated dye inhibitors for malaria parasite triosephosphate isomerase.
|
| |
Proteins,
45,
136-143.
|
 |
|
|
|
|
 |
G.P.Miller,
D.C.Wahnon,
and
S.J.Benkovic
(2001).
Interloop contacts modulate ligand cycling during catalysis by Escherichia coli dihydrofolate reductase.
|
| |
Biochemistry,
40,
867-875.
|
 |
|
|
|
|
 |
H.Reyes-Vivas,
G.Hernández-Alcantara,
G.López-Velazquez,
N.Cabrera,
R.Pérez-Montfort,
M.T.de Gómez-Puyou,
and
A.Gómez-Puyou
(2001).
Factors that control the reactivity of the interface cysteine of triosephosphate isomerase from Trypanosoma brucei and Trypanosoma cruzi.
|
| |
Biochemistry,
40,
3134-3140.
|
 |
|
|
|
|
 |
I.Kursula,
S.Partanen,
A.M.Lambeir,
D.M.Antonov,
K.Augustyns,
and
R.K.Wierenga
(2001).
Structural determinants for ligand binding and catalysis of triosephosphate isomerase.
|
| |
Eur J Biochem,
268,
5189-5196.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Erlandsen,
E.E.Abola,
and
R.C.Stevens
(2000).
Combining structural genomics and enzymology: completing the picture in metabolic pathways and enzyme active sites.
|
| |
Curr Opin Struct Biol,
10,
719-730.
|
 |
|
|
|
|
 |
D.Maes,
J.P.Zeelen,
N.Thanki,
N.Beaucamp,
M.Alvarez,
M.H.Thi,
J.Backmann,
J.A.Martial,
L.Wyns,
R.Jaenicke,
and
R.K.Wierenga
(1999).
The crystal structure of triosephosphate isomerase (TIM) from Thermotoga maritima: a comparative thermostability structural analysis of ten different TIM structures.
|
| |
Proteins,
37,
441-453.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.Saadat,
and
D.H.Harrison
(1999).
The crystal structure of methylglyoxal synthase from Escherichia coli.
|
| |
Structure,
7,
309-317.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Alvarez,
J.Wouters,
D.Maes,
V.Mainfroid,
F.Rentier-Delrue,
L.Wyns,
E.Depiereux,
and
J.A.Martial
(1999).
Lys13 plays a crucial role in the functional adaptation of the thermophilic triose-phosphate isomerase from Bacillus stearothermophilus to high temperatures.
|
| |
J Biol Chem,
274,
19181-19187.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.Pérez-Montfort,
G.Garza-Ramos,
G.H.Alcántara,
H.Reyes-Vivas,
X.G.Gao,
E.Maldonado,
M.T.de Gómez-Puyou,
and
A.Gómez-Puyou
(1999).
Derivatization of the interface cysteine of triosephosphate isomerase from Trypanosoma brucei and Trypanosoma cruzi as probe of the interrelationship between the catalytic sites and the dimer interface.
|
| |
Biochemistry,
38,
4114-4120.
|
 |
|
|
|
|
 |
S.M.King,
and
W.C.Johnson
(1999).
Assigning secondary structure from protein coordinate data.
|
| |
Proteins,
35,
313-320.
|
 |
|
|
|
|
 |
X.G.Gao,
E.Maldonado,
R.Pérez-Montfort,
G.Garza-Ramos,
M.T.de Gómez-Puyou,
A.Gómez-Puyou,
and
A.Rodríguez-Romero
(1999).
Crystal structure of triosephosphate isomerase from Trypanosoma cruzi in hexane.
|
| |
Proc Natl Acad Sci U S A,
96,
10062-10067.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Z.Zhang,
E.A.Komives,
S.Sugio,
S.C.Blacklow,
N.Narayana,
N.H.Xuong,
A.M.Stock,
G.A.Petsko,
and
D.Ringe
(1999).
The role of water in the catalytic efficiency of triosephosphate isomerase.
|
| |
Biochemistry,
38,
4389-4397.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.S.Bell,
R.J.Russell,
M.Kohlhoff,
R.Hensel,
M.J.Danson,
D.W.Hough,
and
G.L.Taylor
(1998).
Preliminary crystallographic studies of triosephosphate isomerase (TIM) from the hyperthermophilic Archaeon Pyrococcus woesei.
|
| |
Acta Crystallogr D Biol Crystallogr,
54,
1419-1421.
|
 |
|
|
|
|
 |
J.Sun,
and
N.S.Sampson
(1998).
Determination of the amino acid requirements for a protein hinge in triosephosphate isomerase.
|
| |
Protein Sci,
7,
1495-1505.
|
 |
|
|
|
|
 |
M.Alvarez,
J.P.Zeelen,
V.Mainfroid,
F.Rentier-Delrue,
J.A.Martial,
L.Wyns,
R.K.Wierenga,
and
D.Maes
(1998).
Triose-phosphate isomerase (TIM) of the psychrophilic bacterium Vibrio marinus. Kinetic and structural properties.
|
| |
J Biol Chem,
273,
2199-2206.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.Derreumaux,
and
T.Schlick
(1998).
The loop opening/closing motion of the enzyme triosephosphate isomerase.
|
| |
Biophys J,
74,
72-81.
|
 |
|
|
|
|
 |
R.Gilboa,
A.J.Bauer,
and
G.Shoham
(1998).
Crystallization and preliminary crystallographic analysis of glyceraldehyde 3-phosphate dehydrogenase from Sacchromyces cerevisiae (baker's yeast).
|
| |
Acta Crystallogr D Biol Crystallogr,
54,
1467-1470.
|
 |
|
|
|
|
 |
T.K.Harris,
R.N.Cole,
F.I.Comer,
and
A.S.Mildvan
(1998).
Proton transfer in the mechanism of triosephosphate isomerase.
|
| |
Biochemistry,
37,
16828-16838.
|
 |
|
|
|
|
 |
A.Landa,
A.Rojo-Domínguez,
L.Jiménez,
and
D.A.Fernández-Velasco
(1997).
Sequencing, expression and properties of triosephosphate isomerase from Entamoeba histolytica.
|
| |
Eur J Biochem,
247,
348-355.
|
 |
|
|
|
|
 |
N.Beaucamp,
A.Hofmann,
B.Kellerer,
and
R.Jaenicke
(1997).
Dissection of the gene of the bifunctional PGK-TIM fusion protein from the hyperthermophilic bacterium Thermotoga maritima: design and characterization of the separate triosephosphate isomerase.
|
| |
Protein Sci,
6,
2159-2165.
|
 |
|
|
|
|
 |
S.S.Velanker,
S.S.Ray,
R.S.Gokhale,
S.Suma,
H.Balaram,
P.Balaram,
and
M.R.Murthy
(1997).
Triosephosphate isomerase from Plasmodium falciparum: the crystal structure provides insights into antimalarial drug design.
|
| |
Structure,
5,
751-761.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.W.Rietveld,
and
S.T.Ferreira
(1996).
Deterministic pressure dissociation and unfolding of triose phosphate isomerase: persistent heterogeneity of a protein dimer.
|
| |
Biochemistry,
35,
7743-7751.
|
 |
|
|
|
|
 |
B.L.Stoddard
(1996).
Intermediate trapping and laue X-ray diffraction: potential for enzyme mechanism, dynamics, and inhibitor screening.
|
| |
Pharmacol Ther,
70,
215-256.
|
 |
|
|
|
|
 |
E.A.Komives,
J.C.Lougheed,
Z.Zhang,
S.Sugio,
N.Narayana,
N.H.Xuong,
G.A.Petsko,
and
D.Ringe
(1996).
The structural basis for pseudoreversion of the H95N lesion by the secondary S96P mutation in triosephosphate isomerase.
|
| |
Biochemistry,
35,
15474-15484.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
F.Takusagawa,
S.Kamitori,
and
G.D.Markham
(1996).
Structure and function of S-adenosylmethionine synthetase: crystal structures of S-adenosylmethionine synthetase with ADP, BrADP, and PPi at 28 angstroms resolution.
|
| |
Biochemistry,
35,
2586-2596.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
F.Takusagawa,
S.Kamitori,
S.Misaki,
and
G.D.Markham
(1996).
Crystal structure of S-adenosylmethionine synthetase.
|
| |
J Biol Chem,
271,
136-147.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
G.Garza-Ramos,
R.Pérez-Montfort,
A.Rojo-Domínguez,
M.T.de Gómez-Puyou,
and
A.Gómez-Puyou
(1996).
Species-specific inhibition of homologous enzymes by modification of nonconserved amino acids residues. The cysteine residues of triosephosphate isomerase.
|
| |
Eur J Biochem,
241,
114-120.
|
 |
|
|
|
|
 |
J.Aqvist,
and
M.Fothergill
(1996).
Computer simulation of the triosephosphate isomerase catalyzed reaction.
|
| |
J Biol Chem,
271,
10010-10016.
|
 |
|
|
|
|
 |
M.Watanabe,
B.C.Zingg,
and
H.W.Mohrenweiser
(1996).
Molecular analysis of a series of alleles in humans with reduced activity at the triosephosphate isomerase locus.
|
| |
Am J Hum Genet,
58,
308-316.
|
 |
|
|
|
|
 |
N.S.Blom,
S.Tétreault,
R.Coulombe,
and
J.Sygusch
(1996).
Novel active site in Escherichia coli fructose 1,6-bisphosphate aldolase.
|
| |
Nat Struct Biol,
3,
856-862.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Janecek
(1996).
Invariant glycines and prolines flanking in loops the strand beta 2 of various (alpha/beta)8-barrel enzymes: a hidden homology?
|
| |
Protein Sci,
5,
1136-1143.
|
 |
|
|
|
|
 |
V.Mainfroid,
S.C.Mande,
W.G.Hol,
J.A.Martial,
and
K.Goraj
(1996).
Stabilization of human triosephosphate isomerase by improvement of the stability of individual alpha-helices in dimeric as well as monomeric forms of the protein.
|
| |
Biochemistry,
35,
4110-4117.
|
 |
|
|
|
|
 |
W.C.Alston,
M.Kanska,
and
C.J.Murray
(1996).
Secondary H/T and D/T isotope effects in enzymatic enolization reactions. Coupled motion and tunneling in the triosephosphate isomerase reaction.
|
| |
Biochemistry,
35,
12873-12881.
|
 |
|
|
|
|
 |
A.D.Cameron,
I.Sinning,
G.L'Hermite,
B.Olin,
P.G.Board,
B.Mannervik,
and
T.A.Jones
(1995).
Structural analysis of human alpha-class glutathione transferase A1-1 in the apo-form and in complexes with ethacrynic acid and its glutathione conjugate.
|
| |
Structure,
3,
717-727.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Gómez-Puyou,
E.Saavedra-Lira,
I.Becker,
R.A.Zubillaga,
A.Rojo-Domínguez,
and
R.Pérez-Montfort
(1995).
Using evolutionary changes to achieve species-specific inhibition of enzyme action--studies with triosephosphate isomerase.
|
| |
Chem Biol,
2,
847-855.
|
 |
|
|
|
|
 |
H.Schurig,
N.Beaucamp,
R.Ostendorp,
R.Jaenicke,
E.Adler,
and
J.R.Knowles
(1995).
Phosphoglycerate kinase and triosephosphate isomerase from the hyperthermophilic bacterium Thermotoga maritima form a covalent bifunctional enzyme complex.
|
| |
EMBO J,
14,
442-451.
|
 |
|
|
|
|
 |
L.F.Delboni,
S.C.Mande,
F.Rentier-Delrue,
V.Mainfroid,
S.Turley,
F.M.Vellieux,
J.A.Martial,
and
W.G.Hol
(1995).
Crystal structure of recombinant triosephosphate isomerase from Bacillus stearothermophilus. An analysis of potential thermostability factors in six isomerases with known three-dimensional structures points to the importance of hydrophobic interactions.
|
| |
Protein Sci,
4,
2594-2604.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Barrett,
C.G.Suresh,
S.P.Tolley,
E.J.Dodson,
and
M.A.Hughes
(1995).
The crystal structure of a cyanogenic beta-glucosidase from white clover, a family 1 glycosyl hydrolase.
|
| |
Structure,
3,
951-960.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Madej,
J.F.Gibrat,
and
S.H.Bryant
(1995).
Threading a database of protein cores.
|
| |
Proteins,
23,
356-369.
|
 |
|
|
|
|
 |
T.V.Borchert,
K.V.Kishan,
J.P.Zeelen,
W.Schliebs,
N.Thanki,
R.Abagyan,
R.Jaenicke,
and
R.K.Wierenga
(1995).
Three new crystal structures of point mutation variants of monoTIM: conformational flexibility of loop-1, loop-4 and loop-8.
|
| |
Structure,
3,
669-679.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Y.H.Zhou,
and
M.A.Ragan
(1995).
Cloning and characterization of the nuclear gene and cDNAs for triosephosphate isomerase of the marine red alga Gracilaria verrucosa.
|
| |
Curr Genet,
28,
317-323.
|
 |
|
|
|
|
 |
C.L.Borders,
J.A.Broadwater,
P.A.Bekeny,
J.E.Salmon,
A.S.Lee,
A.M.Eldridge,
and
V.B.Pett
(1994).
A structural role for arginine in proteins: multiple hydrogen bonds to backbone carbonyl oxygens.
|
| |
Protein Sci,
3,
541-548.
|
 |
|
|
|
|
 |
C.L.Verlinde,
E.A.Merritt,
F.Van den Akker,
H.Kim,
I.Feil,
L.F.Delboni,
S.C.Mande,
S.Sarfaty,
P.H.Petra,
and
W.G.Hol
(1994).
Protein crystallography and infectious diseases.
|
| |
Protein Sci,
3,
1670-1686.
|
 |
|
|
|
|
 |
H.Kono,
and
J.Doi
(1994).
Energy minimization method using automata network for sequence and side-chain conformation prediction from given backbone geometry.
|
| |
Proteins,
19,
244-255.
|
 |
|
|
|
|
 |
K.V.Kishan,
J.P.Zeelen,
M.E.Noble,
T.V.Borchert,
and
R.K.Wierenga
(1994).
Comparison of the structures and the crystal contacts of trypanosomal triosephosphate isomerase in four different crystal forms.
|
| |
Protein Sci,
3,
779-787.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.V.Grishin,
and
M.A.Phillips
(1994).
The subunit interfaces of oligomeric enzymes are conserved to a similar extent to the overall protein sequences.
|
| |
Protein Sci,
3,
2455-2458.
|
 |
|
|
|
|
 |
S.C.Mande,
V.Mainfroid,
K.H.Kalk,
K.Goraj,
J.A.Martial,
and
W.G.Hol
(1994).
Crystal structure of recombinant human triosephosphate isomerase at 2.8 A resolution. Triosephosphate isomerase-related human genetic disorders and comparison with the trypanosomal enzyme.
|
| |
Protein Sci,
3,
810-821.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.A.Schreuder,
S.Knight,
P.M.Curmi,
I.Andersson,
D.Cascio,
C.I.Brändén,
and
D.Eisenberg
(1993).
Formation of the active site of ribulose-1,5-bisphosphate carboxylase/oxygenase by a disorder-order transition from the unactivated to the activated form.
|
| |
Proc Natl Acad Sci U S A,
90,
9968-9972.
|
 |
|
|
|
|
 |
M.E.Noble,
J.P.Zeelen,
and
R.K.Wierenga
(1993).
Structures of the "open" and "closed" state of trypanosomal triosephosphate isomerase, as observed in a new crystal form: implications for the reaction mechanism.
|
| |
Proteins,
16,
311-326.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.L.Chang,
P.J.Artymiuk,
X.Wu,
S.Hollán,
A.Lammi,
and
L.E.Maquat
(1993).
Human triosephosphate isomerase deficiency resulting from mutation of Phe-240.
|
| |
Am J Hum Genet,
52,
1260-1269.
|
 |
|
|
|
|
 |
R.C.Wade,
M.E.Davis,
B.A.Luty,
J.D.Madura,
and
J.A.McCammon
(1993).
Gating of the active site of triose phosphate isomerase: Brownian dynamics simulations of flexible peptide loops in the enzyme.
|
| |
Biophys J,
64,
9.
|
 |
|
|
|
|
 |
S.Janecek,
and
S.Baláz
(1993).
Evolution of parallel beta/alpha-barrel enzyme family lightened by structural data on starch-processing enzymes.
|
| |
J Protein Chem,
12,
509-514.
|
 |
|
|
|
|
 |
T.V.Borchert,
K.Pratt,
J.P.Zeelen,
M.Callens,
M.E.Noble,
F.R.Opperdoes,
P.A.Michels,
and
R.K.Wierenga
(1993).
Overexpression of trypanosomal triosephosphate isomerase in Escherichia coli and characterisation of a dimer-interface mutant.
|
| |
Eur J Biochem,
211,
703-710.
|
 |
|
|
|
|
 |
A.E.Howard,
and
P.A.Kollman
(1992).
Molecular dynamics studies of a DNA-binding protein: 1. A comparison of the trp repressor and trp aporepressor aqueous simulations.
|
| |
Protein Sci,
1,
1173-1184.
|
 |
|
|
|
|
 |
B.A.Perry,
and
H.W.Mohrenweiser
(1992).
Human triosephosphate isomerase: substitution of Arg for Gly at position 122 in a thermolabile electromorph variant, TPI-Manchester.
|
| |
Hum Genet,
88,
634-638.
|
 |
|
|
|
|
 |
G.Garza-Ramos,
M.Tuena de Gómez-Puyou,
A.Gómez-Puyou,
and
R.W.Gracy
(1992).
Dimerization and reactivation of triosephosphate isomerase in reverse micelles.
|
| |
Eur J Biochem,
208,
389-395.
|
 |
|
|
|
|
 |
M.D.Walkinshaw
(1992).
Protein targets for structure-based drug design.
|
| |
Med Res Rev,
12,
317-372.
|
 |
|
|
|
|
 |
R.K.Wierenga,
T.V.Borchert,
and
M.E.Noble
(1992).
Crystallographic binding studies with triosephosphate isomerases: conformational changes induced by substrate and substrate-analogues.
|
| |
FEBS Lett,
307,
34-39.
|
 |
|
|
|
|
 |
K.D.Schnackerz,
and
R.W.Gracy
(1991).
Probing the catalytic sites of triosephosphate isomerase by 31P-NMR with reversibly and irreversibly binding substrate analogues.
|
| |
Eur J Biochem,
199,
231-238.
|
 |
|
|
|
|
 |
M.E.Noble,
R.K.Wierenga,
A.M.Lambeir,
F.R.Opperdoes,
A.M.Thunnissen,
K.H.Kalk,
H.Groendijk,
and
W.G.Hol
(1991).
The adaptability of the active site of trypanosomal triosephosphate isomerase as observed in the crystal structures of three different complexes.
|
| |
Proteins,
10,
50-69.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.K.Wierenga,
M.E.Noble,
J.P.Postma,
H.Groendijk,
K.H.Kalk,
W.G.Hol,
and
F.R.Opperdoes
(1991).
The crystal structure of the "open" and the "closed" conformation of the flexible loop of trypanosomal triosephosphate isomerase.
|
| |
Proteins,
10,
33-49.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

