 |
PDBsum entry 1m7y

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.4.1.14
- 1-aminocyclopropane-1-carboxylate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Ethylene Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
S-adenosyl-L-methionine = 1-aminocyclopropane-1-carboxylate + S-methyl- 5'-thioadenosine + H+
|
 |
 |
 |
 |
 |
 S-adenosyl-L-methionine
S-adenosyl-L-methionine
|
=
|
1-aminocyclopropane-1-carboxylate
Bound ligand (Het Group name = )
matches with 50.00% similarity
|
+
|
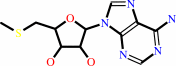 S-methyl- 5'-thioadenosine
S-methyl- 5'-thioadenosine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PPG)
matches with 55.56% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
277:49735-49742
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Apple 1-aminocyclopropane-1-carboxylate synthase in complex with the inhibitor L-aminoethoxyvinylglycine. Evidence for a ketimine intermediate.
|
|
G.Capitani,
D.L.McCarthy,
H.Gut,
M.G.Grütter,
J.F.Kirsch.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The 1.6-A crystal structure of the covalent ketimine complex of apple
1-aminocyclopropane-1-carboxylate (ACC) synthase with the potent inhibitor
l-aminoethoxyvinylglycine (AVG) is described. ACC synthase catalyzes the
committed step in the biosynthesis of ethylene, a plant hormone that is
responsible for the initiation of fruit ripening and for regulating many other
developmental processes. AVG is widely used in plant physiology studies to
inhibit the activity of ACC synthase. The structural assignment is supported by
the fact that the complex absorbs maximally at 341 nm. These results are not in
accord with the recently reported crystal structure of the tomato ACC synthase
AVG complex, which claims that the inhibitor only associates noncovalently. The
rate constant for the association of AVG with apple ACC synthase was determined
by stopped-flow spectrophotometry (2.1 x 10(5) m(-1) s(-1)) and by the rate of
loss of enzyme activity (1.1 x 10(5) m(-1) s(-1)). The dissociation rate
constant determined by activity recovery is 2.4 x 10(-6) s(-1). Thus, the
calculated K(d) value is 10-20 pm.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 6.
Fig. 6. A, superposition of the active sites of
unliganded (sea green) and AVG-bound (yellow) ACC synthase. Only
selected residues are shown. B, the active sites of AVG-bound
ACC synthase (gray) and cystathionine  -lyase (sea
green). C, the C -lyase (sea
green). C, the C  traces of
unliganded (yellow) and AVG-bound (blue) ACC synthase. This
illustration was prepared with DINO. traces of
unliganded (yellow) and AVG-bound (blue) ACC synthase. This
illustration was prepared with DINO.
|
 |
Figure 7.
Fig. 7. LIGPLOT (13) representation of the hydrogen
bonding between ACC synthase and the AVG·PLP adduct.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2002,
277,
49735-49742)
copyright 2002.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.R.Choudhury,
S.K.Singh,
S.Roy,
and
D.N.Sengupta
(2010).
An insight into the sequential, structural and phylogenetic properties of banana 1-aminocyclopropane-1-carboxylate synthase 1 and study of its interaction with pyridoxal-5'-phosphate and aminoethoxyvinylglycine.
|
| |
J Biosci,
35,
281-294.
|
 |
|
|
|
|
 |
A.Thomann,
E.Lechner,
M.Hansen,
E.Dumbliauskas,
Y.Parmentier,
J.Kieber,
B.Scheres,
and
P.Genschik
(2009).
Arabidopsis CULLIN3 genes regulate primary root growth and patterning by ethylene-dependent and -independent mechanisms.
|
| |
PLoS Genet,
5,
e1000328.
|
 |
|
|
|
|
 |
A.Danon,
O.Miersch,
G.Felix,
R.G.Camp,
and
K.Apel
(2005).
Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana.
|
| |
Plant J,
41,
68-80.
|
 |
|
|
|
|
 |
A.C.Eliot,
and
J.F.Kirsch
(2004).
Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations.
|
| |
Annu Rev Biochem,
73,
383-415.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

